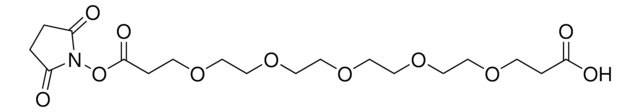
803200
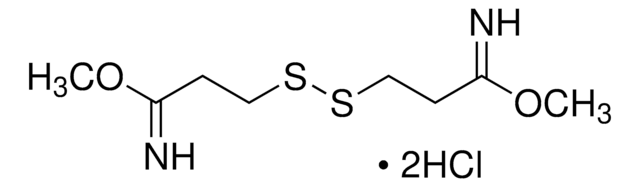
DTSSP (3,3′-dithiobis(sulfosuccinimidyl propionate))
Synonym(s):
DTSSP, bis(sulfosuccinimidyl) 3,3′-dithiobis(propionate)
About This Item
Recommended Products
Assay
≥80%
Quality Level
form
powder
mol wt
608.51
reaction suitability
reagent type: cross-linking reagent
storage condition
desiccated
solubility
soluble (>6mg/ml in water)
shipped in
ambient
storage temp.
2-8°C
SMILES string
O=C(CC1S(=O)([O-])=O)N(OC(CCSSCCC(ON2C(CC(S(=O)([O-])=O)C2=O)=O)=O)=O)C1=O.[Na+].[Na+]
InChI
1S/C14H16N2O14S4.2Na/c17-9-5-7(33(23,24)25)13(21)15(9)29-11(19)1-3-31-32-4-2-12(20)30-16-10(18)6-8(14(16)22)34(26,27)28;;/h7-8H,1-6H2,(H,23,24,25)(H,26,27,28);;/q;2*+1/p-2
InChI key
XRWWBWZHHSXBNC-UHFFFAOYSA-L
Related Categories
General description
Application
- Enhancing Ocular Antisense Oligonucleotide Delivery: Utilizing Cowpea Chlorotic Mottle Virus-like particles, this study incorporates DTSSP for the enhancement of antisense oligonucleotide delivery targeting posterior segment ocular diseases, demonstrating a novel approach in biocompatible drug delivery systems (Pretto et al., 2021).
- Regulation of Integrin-Tetraspanin Interactions: DTSSP is utilized to stabilize interactions between integrins and the tetraspanin CD9, providing critical insights into cell adhesion and signaling mechanisms, relevant to cancer and other pathologies (Torres-Gómez et al., 2021).
- Gene Delivery Platform Development: A study on adeno-associated viral vectors employs DTSSP for cross-linking to tune cellular tropisms and enhance gene delivery, showing potential for targeted gene therapy applications (Yoo et al., 2020).
- Protein Interactor Identification: DTSSP is instrumental in identifying interactors of the Arabidopsis thaliana Plant Natriuretic Peptide (AtPNP-A) using mass spectrometry, aiding in the understanding of plant signaling pathways (Turek et al., 2020).
- Protein Delivery via Nanoparticles: Research on poly(l-lysine)-grafted-poly(ethylene glycol) nanoparticles highlights the use of DTSSP for redox-responsive crosslinking, improving the delivery mechanisms for therapeutic proteins (Seaberg et al., 2020).
Features and Benefits
- Reactive groups: sulfo-NHS ester (both ends)
- Reactive towards: amino groups (primary amines)
- Sulfo-NHS ester reacts rapidly with any primary amine-containing molecule
- Disulfide bond in the spacer arm is readily cleaved by 10-50 mM DTT or TCEP at pH 8.5
- Spacer arm also easily cleaved with reducing SDS-PAGE sample loading
- Cleavable crosslinker allows separation of crosslinked products
- Water-soluble; compare with DSP
- Membrane-impermeable, allowing for cell surface labeling
Caution
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service


![LC-SPDP (succinimidyl 6-[3(2-pyridyldithio)propionamido]hexanoate)](/deepweb/assets/sigmaaldrich/product/structures/300/586/d95fd80c-e201-4b0b-8aee-31e109c2ff41/640/d95fd80c-e201-4b0b-8aee-31e109c2ff41.png)