742937
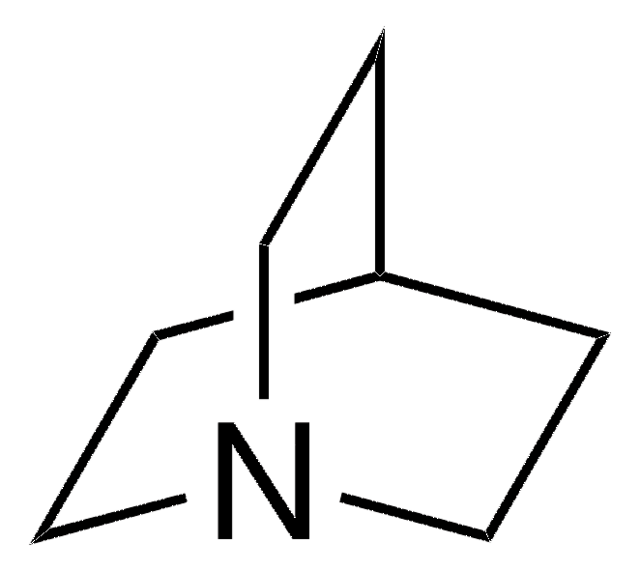
1,4-Diazabicyclo[2.2.2]octane bis(sulfur dioxide) adduct
≥95% (sulfur, elemental analysis)
Synonym(s):
DABCO•(SO2)2, DABSO
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H12N2O4S2
CAS Number:
Molecular Weight:
240.30
MDL number:
UNSPSC Code:
12352005
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥95% (sulfur, elemental analysis)
form
powder
composition
active SO2, ~50%
carbon, 28.3-31.6%
storage temp.
2-8°C
SMILES string
[O-]S(=O)[N+]12CC[N+](CC1)(CC2)S([O-])=O
InChI
1S/C6H12N2O4S2/c9-13(10)7-1-2-8(5-3-7,6-4-7)14(11)12/h1-6H2
InChI key
RWISEVUOFYXWFO-UHFFFAOYSA-N
Application
1,4-Diazabicyclo[2.2.2]octane bis(sulfur dioxide) (DABSO) is a charge transfer complex that can be used as a sulfur dioxide surrogate:
It can also be used to activate DMSO and o-vinylanilines for the synthesis of N-aryl-1H-benzo[d]imidazol-1-amine and 4-aryl quinolines, respectively.
- In palladium-catalyzed aminosulfonylation process.
- In reaction with aryl bromides to synthesize sodium aryl sulfinates.
It can also be used to activate DMSO and o-vinylanilines for the synthesis of N-aryl-1H-benzo[d]imidazol-1-amine and 4-aryl quinolines, respectively.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Chronic 3 - Eye Irrit. 2 - Flam. Sol. 1 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
4.1B - Flammable solid hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Bao Nguyen et al.
Journal of the American Chemical Society, 132(46), 16372-16373 (2010-10-30)
The palladium-catalyzed three-component coupling of aryl iodides, sulfur dioxide, and hydrazines to deliver aryl N-aminosulfonamides is described. The colorless crystalline solid DABCO·(SO(2))(2) was used as a convenient source of sulfur dioxide. The reaction tolerates significant variation of both the aryl
Synthesis of sodium aryl sulfinates from aryl bromides employing 1, 4-diazabicyclo [2.2. 2] octane bis (sulfur dioxide) adduct (DABSO) as a bench-stable, gas-free alternative to SO2.
Skillinghaug B, et al.
Tetrahedron Letters, 57(5), 533-536 (2016)
Carbon annulation of ortho-vinylanilines with dimethyl sulfoxide to access 4-aryl quinolines.
Yuan J, et al.
Organic & Biomolecular Chemistry, 15(6), 1334-1337 (2017)
Palladium-catalyzed aminosulfonylation of aryl halides.
Nguyen B, et al.
Journal of the American Chemical Society, 132(46), 16372-16373 (2010)
Palladium-catalyzed annulation of 2-(aryldiazenyl) aniline with dimethyl sulfoxide to access N-aryl-1H-benzo [d] imidazol-1-amine.
Wang H, et al.
Tetrahedron Letters, 58(40), 3875-3878 (2017)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service![1,4-Diazabicyclo[2.2.2]octane ReagentPlus®, ≥99%](/deepweb/assets/sigmaaldrich/product/structures/366/129/a6ff4175-974d-4fac-9038-b35e508ef252/640/a6ff4175-974d-4fac-9038-b35e508ef252.png)
![1,8-Diazabicyclo[5.4.0]undec-7-ene 98%](/deepweb/assets/sigmaaldrich/product/structures/120/564/5b373e23-1624-489c-8efb-692de0f96ffb/640/5b373e23-1624-489c-8efb-692de0f96ffb.png)
![Bis(trimethylaluminum)-1,4-diazabicyclo[2.2.2]octane adduct](/deepweb/assets/sigmaaldrich/product/structures/978/293/6c8c7fbe-4b40-4576-bd40-94ac58cbe057/640/6c8c7fbe-4b40-4576-bd40-94ac58cbe057.png)