698253
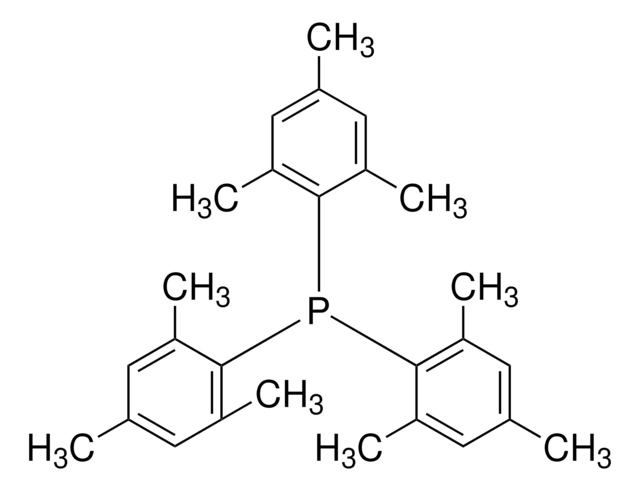
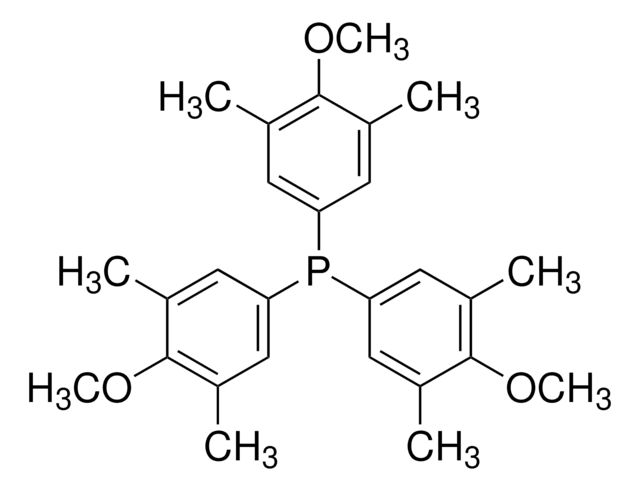
Tris(3,5-dimethylphenyl)phosphine
96%
Synonym(s):
Tri-3,5-xylylphosphine
About This Item
Recommended Products
Assay
96%
form
powder
reaction suitability
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reaction type: Heck Reaction
reaction type: Hiyama Coupling
reaction type: Negishi Coupling
reaction type: Sonogashira Coupling
reaction type: Stille Coupling
reaction type: Suzuki-Miyaura Coupling
reagent type: ligand
mp
160-165 °C
functional group
phosphine
SMILES string
Cc1cc(C)cc(c1)P(c2cc(C)cc(C)c2)c3cc(C)cc(C)c3
InChI
1S/C24H27P/c1-16-7-17(2)11-22(10-16)25(23-12-18(3)8-19(4)13-23)24-14-20(5)9-21(6)15-24/h7-15H,1-6H3
InChI key
XRALRSQLQXKXKP-UHFFFAOYSA-N
Related Categories
Application
- Cu / diphosphine-catalyzed asymmetric hydrogenation of heteroaromatic ketones and enones
- Highly selective rhodium-catalyzed hydrogenation reactions
- Classical versus kinetic resolution in preparation of privileged silicon-stereogenic silanaphthalenes
Used for kinetic resolution of donor-functionalized secondary alcohols via Cu-H-catalyzed stereoselective silylation by dehydrogenative Si-O coupling with Si-stereogenic silanes
Used for comparative studies of conformational rigidity of silicon-stereogenic silanes in asymmetric catalysis
Signal Word
Warning
Hazard Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service