All Photos(1)
About This Item
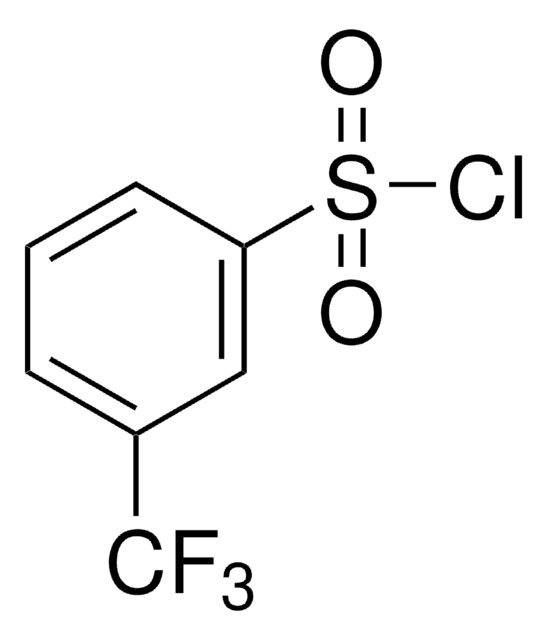
Linear Formula:
CH3OC6H4SO2Cl
CAS Number:
Molecular Weight:
206.65
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
96%
refractive index
n20/D 1.5560 (lit.)
density
1.460 g/mL at 25 °C (lit.)
SMILES string
COc1cccc(c1)S(Cl)(=O)=O
InChI
1S/C7H7ClO3S/c1-11-6-3-2-4-7(5-6)12(8,9)10/h2-5H,1H3
InChI key
JHJKSEKUZNJKGO-UHFFFAOYSA-N
Application
3-Methoxybenzenesulfonyl chloride may be used to synthesize 3-(4-phenylpiperazin-1yl) sulfonyls and N-(1-(4-methoxybenzyl)-3-cyclopropyl-1Hpyrazol-5-yl)-3-methoxybenzenesulfonamide.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Addressing cytotoxicity of 1, 4-biphenyl amide derivatives: Discovery of new potent and selective 17b-hydroxysteroid dehydrogenase type 2 inhibitors.
Gargano EM, et al.
Bioorganic & Medicinal Chemistry Letters (2015)
Hanumegowda Raju et al.
Recent patents on anti-cancer drug discovery, 6(2), 186-195 (2011-01-21)
In search of synthetic chemotherapeutic substances capable of inhibiting, retarding, or reversing the process of multistage carcinogenesis, we synthesised a series of novel 1-(4-methoxybenzyl)-3-cyclopropyl-1H-pyrazol-5-amine derivatives 9(a-h) by a nucleophilic substitution reaction and characterized by (1)H and (13)C nuclear magnetic resonance
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service