47695
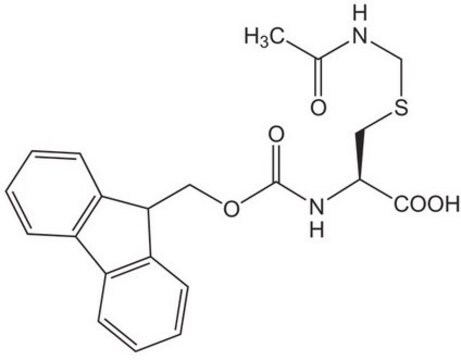
Fmoc-Cys(Trt)-OH
≥95.0% (sum of enantiomers, HPLC)
Synonym(s):
N-(9-Fluorenylmethoxycarbonyl)-S-trityl-L-cysteine, Nα-Fmoc-S-trityl-L-cysteine
About This Item
Recommended Products
Quality Level
Assay
≥95.0% (sum of enantiomers, HPLC)
optical activity
[α]20/D +16.0±2°, c = 1% in THF
reaction suitability
reaction type: Fmoc solid-phase peptide synthesis
mp
170-173 °C (lit.)
application(s)
peptide synthesis
functional group
Fmoc
storage temp.
2-8°C
SMILES string
OC(=O)[C@H](CSC(c1ccccc1)(c2ccccc2)c3ccccc3)NC(=O)OCC4c5ccccc5-c6ccccc46
InChI
1S/C37H31NO4S/c39-35(40)34(38-36(41)42-24-33-31-22-12-10-20-29(31)30-21-11-13-23-32(30)33)25-43-37(26-14-4-1-5-15-26,27-16-6-2-7-17-27)28-18-8-3-9-19-28/h1-23,33-34H,24-25H2,(H,38,41)(H,39,40)/t34-/m0/s1
InChI key
KLBPUVPNPAJWHZ-UMSFTDKQSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- Synthesis of mono- and bi-functionalized platinum(IV) complexes to target angiogenic tumor vasculature.
- Synthesis of proteins through native chemical ligation of peptide hydrazides as thioester surrogates via solid-phase synthesis.
- Synthesis of glycoconjugates by conjugating reducing sugars to cysteine residues of peptides.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
With a growing peptide drug market the fast, reliable and uncomplicated synthesis of peptides is of paramount importance.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service