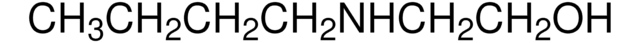471240
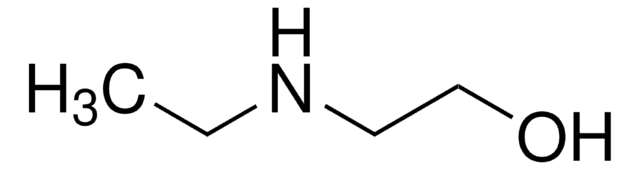
N-Butyldiethanolamine
≥98.6%
Synonym(s):
2,2′-Butyliminodiethanol, N,N-Bis(2-hydroxyethyl)butylamine
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
CH3(CH2)3N(CH2CH2OH)2
CAS Number:
Molecular Weight:
161.24
Beilstein:
1739642
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor density
5.5 (vs air)
Quality Level
vapor pressure
1 mmHg ( 25 °C)
Assay
≥98.6%
refractive index
n20/D 1.463 (lit.)
bp
273-275 °C/741 mmHg (lit.)
mp
−70 °C (lit.)
density
0.986 g/mL at 25 °C (lit.)
SMILES string
CCCCN(CCO)CCO
InChI
1S/C8H19NO2/c1-2-3-4-9(5-7-10)6-8-11/h10-11H,2-8H2,1H3
InChI key
GVNHOISKXMSMPX-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
N-Butyldiethanolamine (N-n-butyldiethanolamine) is a tertiary amine. It acts as an N-substituted diethanolamine ligand. It reacts with chromium(II) and lanthanide(III)/rare earth salts (Ln = La, Ce, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, Lu, Y) in the presence of coligands to afford three series of isostructural 1:1 3d(Cr(III))/4f(Ln(III)) coordination cluster compounds.
Application
N-Butyldiethanolamine (H2bdea, N-n-butyldiethanolamine) has been used in the synthesis of the following complexes (Hdnba = 3,5-dinitrobenzoic acid, Hpta = p-toluic acid, H2tpa = terephthalic acid):
- new mononuclear [Cu(Hbdea)2]·2Hdnba , dinuclear [Cu2(μ-Hbdea)2(N3)2] and [Cu2(μ-Hbdea)2(pta)2]·2H2O
- 1D polymeric [Cu2(μ-Hbdea)2(μ-tpa)]n·2nH2O copper(II) compounds
- tetranuclear 3d-4f single-molecule magnet (SMM) complexes
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1
Storage Class Code
10 - Combustible liquids
WGK
WGK 1
Flash Point(F)
265.1 °F - closed cup
Flash Point(C)
129.5 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Stuart K Langley et al.
Inorganic chemistry, 54(7), 3631-3642 (2015-03-24)
Six tetranuclear 3d–4f single-molecule magnet (SMM) complexes formed using N-n-butyldiethanolamine and N-methyldiethanolamine in conjunction with ortho- and para-substituted benzoic acid and hexafluoroacetoacetone ligands yield two families, both having a butterfly metallic core. The first consists of four complexes of type
Julia Rinck et al.
Inorganic chemistry, 54(7), 3107-3117 (2015-03-11)
Reactions of the N-substituted diethanolamine ligand N-n-butyldiethanolamine with chromium(II) and lanthanide(III)/rare earth salts (Ln = La, Ce, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, Lu, Y) in the presence of coligands give access to three series
Katrin R Gruenwald et al.
Dalton transactions (Cambridge, England : 2003), (12)(12), 2109-2120 (2009-03-11)
The new mononuclear [Cu(Hbdea)(2)].2Hdnba (), dinuclear [Cu(2)(mu-Hbdea)(2)(N(3))(2)] () and [Cu(2)(mu-Hbdea)(2)(pta)(2)].2H(2)O (), and 1D polymeric [Cu(2)(mu-Hbdea)(2)(mu-tpa)](n).2nH(2)O () copper(II) compounds have been prepared by self-assembly, in aqueous alkali medium and at ambient conditions, from Cu(II) acetate, N-butyldiethanolamine (H(2)bdea) and the corresponding auxiliary
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service