All Photos(3)
About This Item
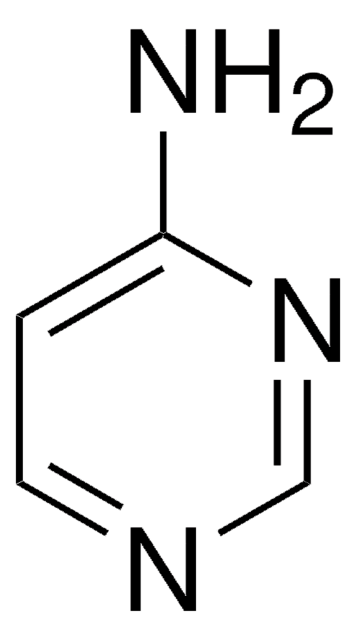
Empirical Formula (Hill Notation):
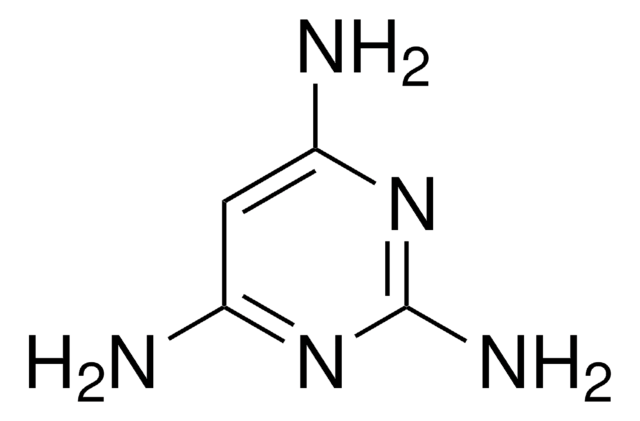
C4H6N4
CAS Number:
Molecular Weight:
110.12
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
mp
143-147 °C (lit.)
SMILES string
Nc1ccnc(N)n1
InChI
1S/C4H6N4/c5-3-1-2-7-4(6)8-3/h1-2H,(H4,5,6,7,8)
InChI key
YAAWASYJIRZXSZ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Hydrogen-bonded supramolecular structures in co-crystals of ?-or ?-diketone enols with 2, 6-diaminopyridine or 2, 4-diaminopyrimidine.
Bertolasi V, et al.
New. J. Chem., 26(11), 1559-1566 (2002)
Wilhelm Maximilian Hützler et al.
Acta crystallographica. Section C, Structural chemistry, 71(Pt 3), 229-238 (2015-03-04)
The results of seven cocrystallization experiments of the antithyroid drug 6-methyl-2-thiouracil (MTU), C(5)H(6)N(2)OS, with 2,4-diaminopyrimidine, 2,4,6-triaminopyrimidine and 6-amino-3H-isocytosine (viz. 2,6-diamino-3H-pyrimidin-4-one) are reported. MTU features an ADA (A = acceptor and D = donor) hydrogen-bonding site, while the three coformers show
Wenbo Zhou et al.
European journal of medicinal chemistry, 96, 269-280 (2015-04-23)
Therapeutics of metastatic or triple-negative breast cancer are still challenging in clinical. Herein we demonstrated the design and optimization of a series of hybrid of 2,4-diaminopyrimidine and arylthiazole derivatives for their anti-proliferative properties against two breast cancer cell lines (MCF-7
Craig A Zificsak et al.
Bioorganic & medicinal chemistry letters, 21(13), 3877-3880 (2011-06-03)
The incorporation of R,R-1,2-diaminocyclohexane at C4 in a series of 2,4-diaminopyrimidines led to a number of ALK inhibitors in which optimized activity was achieved by conversion of the 2-amino group into a methanesulfonamide. Tumor growth inhibition was observed when an
Ilya Lebeau et al.
Antimicrobial agents and chemotherapy, 51(6), 2268-2273 (2007-04-11)
Murine polyomavirus and simian virus 40 were used to evaluate the potencies of the compounds of three classes of acyclic nucleoside phosphonates: (i) the original HPMP (3-hydroxy-2-phosphonomethoxypropyl) and PME (2-phosphonomethoxyethyl) derivatives, (ii) the 6-[2-(phosphonomethoxy)alkoxy]-2,4-diaminopyrimidine (DAPy) derivatives, and (iii) a new
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service