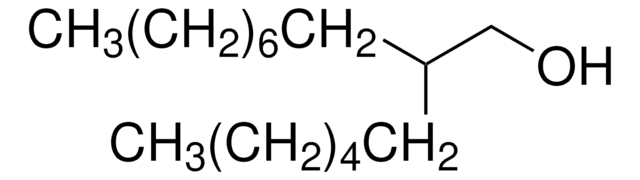464465
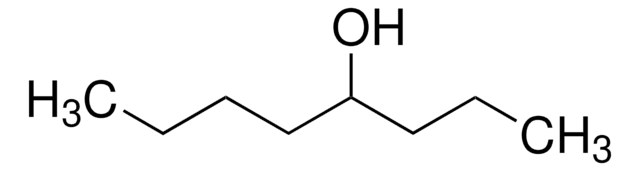
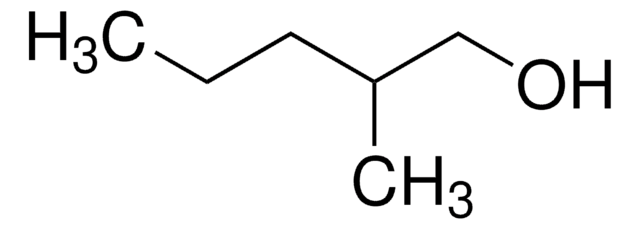
2-Butyl-1-octanol
95%
Synonym(s):
2-Butyloctanol, 2-Butyloctyl alcohol, 5-(Hydroxymethyl)undecane, Butyloctanol, Guerbet dodecanol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3(CH2)5CH[(CH2)3CH3]CH2OH
CAS Number:
Molecular Weight:
186.33
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
95%
bp
145-149 °C (lit.)
density
0.833 g/mL at 25 °C (lit.)
functional group
hydroxyl
SMILES string
CCCCCCC(CO)CCCC
InChI
1S/C12H26O/c1-3-5-7-8-10-12(11-13)9-6-4-2/h12-13H,3-11H2,1-2H3
InChI key
XMVBHZBLHNOQON-UHFFFAOYSA-N
General description
2-Butyl-1-octanol (BuOA) is a long-chain glass forming monohydroxy alcohol.
Application
2-Butyl-1-octanol (BuOA) has been used to synthesize:
It has also been used as an extraction solvent in extractive fed-batch experiments.
- 2-butyl-1-octyl-methacrylate (BOMA)
- 3,5,5-trimethyl-1-hexyl methacrylate (TMHMA)
- hydrophobic polyesters in miniemulsion in the presence of large amounts of water
It has also been used as an extraction solvent in extractive fed-batch experiments.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Acute 1 - Aquatic Chronic 1
Storage Class Code
10 - Combustible liquids
WGK
WGK 2
Flash Point(F)
251.6 °F - Non-equilibrium method
Flash Point(C)
122 °C - Non-equilibrium method
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Polyester synthesis in aqueous miniemulsion.
Barrere M and Landfester K.
Polymer, 44(10), 2833-2841 (2003)
Gergely Kali et al.
Langmuir : the ACS journal of surfaces and colloids, 23(21), 10746-10755 (2007-09-11)
Seven amphiphilic conetworks of methacrylic acid (MAA) and a new hydrophobic monomer, 2-butyl-1-octyl-methacrylate (BOMA), were synthesized using group transfer polymerization. The MAA units were introduced via the polymerization of tetrahydropyranyl methacrylate (THPMA) followed by the removal of the protecting tetrahydropyranyl
Helena González-Peñas et al.
Biotechnology letters, 37(3), 577-584 (2014-10-30)
Acetone/butanol/ethanol (ABE) fermentation by Clostridium acetobutylicum was investigated in extractive fed-batch experiments. In conventional fermentations, metabolic activity ceases when a critical threshold products concentration is reached (~21.6 g solvents l(-1)). Solvents production was increased up to 36.6 and 37.2 g
Yanqin Gao et al.
The Journal of chemical physics, 139(16), 164504-164504 (2013-11-05)
The dielectric relaxation of two long-chain glass forming monohydroxy alcohols, 2-butyl-1-octanol and 2-hexyl-1-decanol, is studied at low temperature. Remarkable broadening from the pure Debye relaxation is identified for the slowest dynamics, differing from the dielectric spectra of short-chain alcohols. The
Shuang Bi et al.
Food chemistry, 289, 680-692 (2019-04-09)
The effects of roasting, boiling, and freeze-drying after boiling on volatile aroma compounds in three varieties of Chinese foxtail millet (Setaria italica), namely, Jingu 21, Fenghonggu and Dongfangliang were determined. During boiling significant (p < 0.05) increases in the contents of several
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service