364037
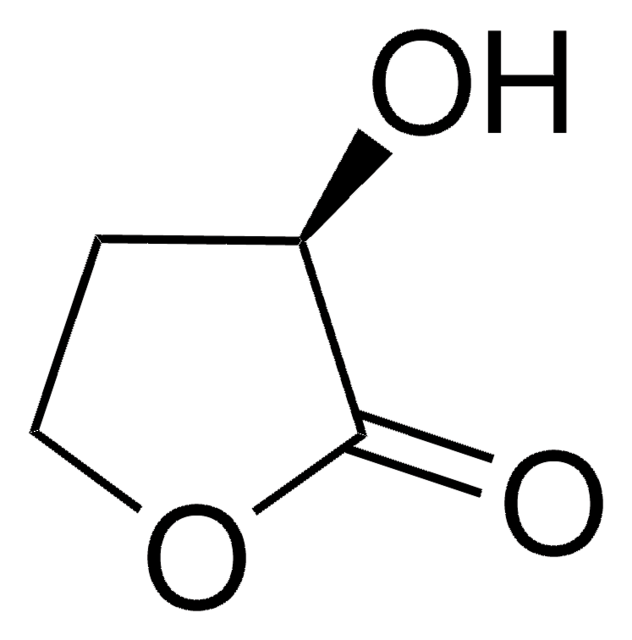
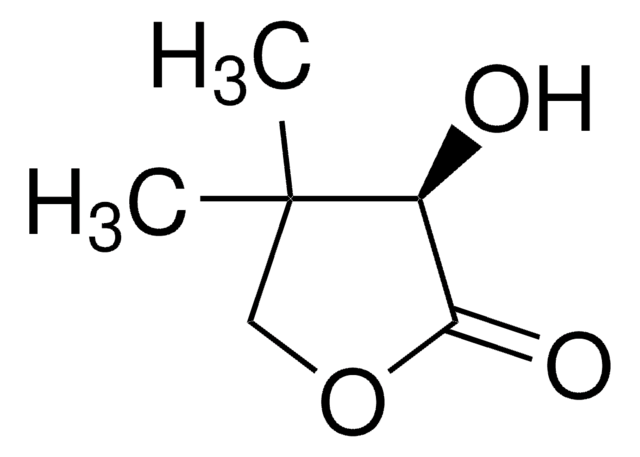
α-Hydroxy-γ-butyrolactone
technical grade
Synonym(s):
4,5-Dihydro-3-hydroxy-2(3H)-furanone
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C4H6O3
CAS Number:
Molecular Weight:
102.09
Beilstein:
80587
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
technical grade
Quality Level
form
viscous liquid
refractive index
n20/D 1.468 (lit.)
bp
133 °C/10 mmHg (lit.)
density
1.309 g/mL at 25 °C (lit.)
SMILES string
OC1CCOC1=O
InChI
1S/C4H6O3/c5-3-1-2-7-4(3)6/h3,5H,1-2H2
InChI key
FWIBCWKHNZBDLS-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
α-Hydroxy-γ-butyrolactone is a 5-membered cyclic ester. It was obtained via tin-conversion of biomass-derived 1,3-dihydroxyacetone (DHA) and formaldehyde.
Application
α-Hydroxy-γ-butyrolactone may be employed as starting reagent in the synthesis of series of seco-pseudonucleoside synthons via aminolysis. It may be employed as starting reagent in the synthesis of enantiomerically pure orthogonally protected δ-azaproline, via Mitsunobu reaction.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Efficient synthesis of enantiomerically pure (S)-d-azaproline starting from (R)-a-hydroxy-?-butyrolactone via the Mitsunobu reaction.
Voss E, et al.
Tetrahedron Asymmetry, 20(15), 1809-1812 (2009)
Sho Yamaguchi et al.
Chemical communications (Cambridge, England), 50(35), 4600-4602 (2014-03-29)
The direct conversion of biomass-derived 1,3-dihydroxyacetone (DHA) and formaldehyde to α-hydroxy-γ-butyrolactone (HBL) was achieved through the use of tin(iv) chloride and a small amount of water and the yield reached up to 70%. The reaction mechanism was also investigated by
Natalia N Dioubankova et al.
Organic letters, 4(26), 4607-4610 (2002-12-20)
[reaction: see text] Two series of seco-pseudonucleoside synthons were synthesized from (R)-(+)-alpha-hydroxy-gamma-butyrolactone and (R)-(-)-pantolactone by aminolysis, side-chain protection, dimethoxytritylation, and phosphitylation or solid-phase attachment. The phosphoramidites and solid supports were used in automated DNA synthesis to prepare oligonucleotides modified with
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service