337358
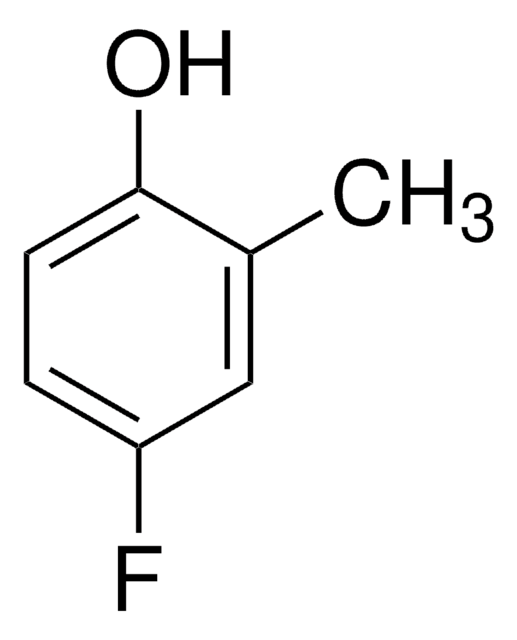
4-Iodo-2-methylphenol
97%
Synonym(s):
4-Iodo-o-cresol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
IC6H3(CH3)OH
CAS Number:
Molecular Weight:
234.03
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
bp
105-110 °C/2 mmHg (lit.)
mp
67-68 °C (lit.)
functional group
iodo
SMILES string
Cc1cc(I)ccc1O
InChI
1S/C7H7IO/c1-5-4-6(8)2-3-7(5)9/h2-4,9H,1H3
InChI key
WSBDSSKIWDFOBQ-UHFFFAOYSA-N
General description
4-Iodo-2-methylphenol was prepared by direct iodination of 2-methylphenol in aqueous alcohol solvents by the action of a reagent prepared in situ from sodium hypochlorite and sodium iodide.
Application
4-Iodo-2-methylphenol was used as starting reagent in the synthesis of an agonist for the peroxisome proliferator-activated receptor δ (PPARδ) GW501516, a potential antiobesity drug.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
An efficient and selective method for the preparation of iodophenols.
Edgar KJ and Falling SN.
The Journal of Organic Chemistry, 55(18), 5287-5291 (1990)
A highly efficient synthesis of antiobestic ligand GW501516 for the peroxisome proliferator-activated receptor d through in situ protection of the phenol group by reaction with a Grignard reagent.
Ham J and Kang H.
Tetrahedron Letters, 46(39), 6683-6686 (2005)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service