All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C12H8N2
CAS Number:
Molecular Weight:
180.21
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
mp
172-174 °C (lit.)
SMILES string
c1cnc2ccc3ncccc3c2c1
InChI
1S/C12H8N2/c1-3-9-10-4-2-8-14-12(10)6-5-11(9)13-7-1/h1-8H
InChI key
DATYUTWESAKQQM-UHFFFAOYSA-N
General description
4,7-Phenanthroline reacts with ruthenium carbonyl to yield cyclometalated derivatives.
Application
4,7-Phenanthroline was used in preparation of:
- cyclic tetranuclear half-sandwich ruthenium(II) complexes
- positively charged homochiral cyclic trinuclear metallacalix[3]arene species
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Julien Frey et al.
Journal of the American Chemical Society, 130(33), 11013-11022 (2008-07-26)
Variously substituted coordinating rigid rods have been synthesized which incorporate a central 4,7-phenanthroline nucleus attached to two 2-pyridyl groups via its 3 and 8 positions, so as to yield bis-bidentate chelates, the two-coordinating axes of the chelates being parallel to
Miguel A Galindo et al.
Journal of inorganic biochemistry, 102(5-6), 1025-1032 (2008-01-12)
The reaction between [(eta(6)-p-cymene)Ru(H2O)3]X2 and 4,7-phenanthroline (phen) leads to the formation of the rectangular tetranuclear complexes [(eta(6)-p-cymene)4Ru4(mu-4,7-phen-N4,N7)(2)(mu-OH)4]X4 (X=NO3, 1a; SO3CF3, 1b) which have been structurally characterised by X-ray crystallography. 1H NMR spectroscopic studies suggest the presence of a partially dissociated
Miguel A Galindo et al.
Dalton transactions (Cambridge, England : 2003), (10)(10), 1563-1566 (2004-07-15)
Reaction of [(dach)Pd(NO3)2] entities (dach = (R,R)-1,2-diaminocyclohexane, (S,S)-1,2-diaminocyclohexane) and 4,7-phenanthroline (phen) providing, respectively, 90 and 120 degrees bond angles, leads to the formation of two novel positively charged homochiral cyclic trinuclear metallacalix[3]arene species [((R,R)-1,2-diaminocyclohexane)Pd(phen)]3(NO3)6 (2a) and [((S,S)-1,2-diaminocyclohexane)Pd(phen)]3(NO3)6 (2b). These species
L W Mitchell et al.
Archives of biochemistry and biophysics, 300(1), 169-177 (1993-01-01)
Porphobilinogen synthase (PBGS) is essential to all life forms; in mammals it is definitively established that Zn(II) is required for activity. The literature regarding the metal requirement for PBGS in other animals, plants, and bacteria neither establishes nor disproves a
V Arluison et al.
Biochemistry, 37(20), 7268-7276 (1998-06-04)
RNA:pseudouridine synthetase (Pus1) from Saccharomyces cerevisiae is a multisite specific enzyme that catalyzes the formation of pseudouridine at positions 34 and 36 of intron-containing precursor tRNAIle and at positions 27 and/or 28 of several yeast tRNAs. In this paper we
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service

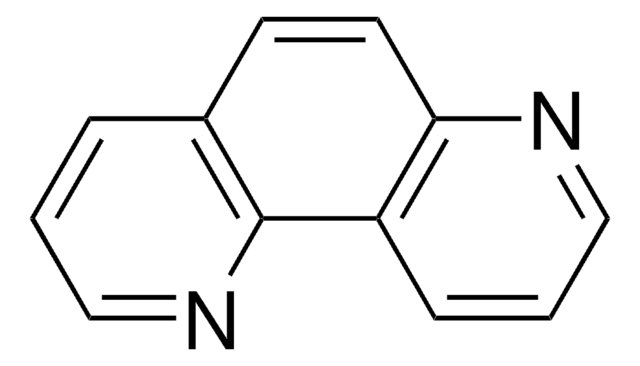
![Pyrazino[2,3-f][1,10]phenanthroline 99% (HPLC)](/deepweb/assets/sigmaaldrich/product/structures/226/341/31d3909e-6700-4a3e-bfb3-9ed1f6b66ee2/640/31d3909e-6700-4a3e-bfb3-9ed1f6b66ee2.png)