301558
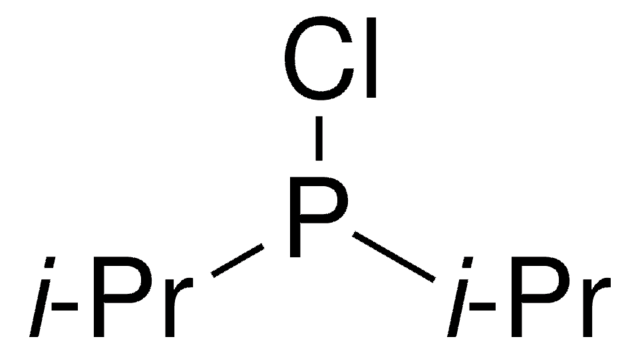
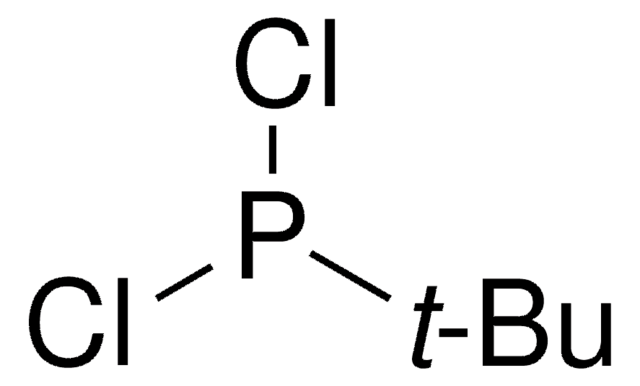
Di-tert-butylchlorophosphine
96%
Synonym(s):
P, P-bis(1 1-dimethylethyl), Phosphinous chloride
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
[(CH3)3C]2PCl
CAS Number:
Molecular Weight:
180.66
MDL number:
UNSPSC Code:
12352001
PubChem Substance ID:
Recommended Products
Quality Level
Assay
96%
form
liquid
reaction suitability
reagent type: ligand
reaction type: Suzuki-Miyaura Coupling
refractive index
n20/D 1.482 (lit.)
bp
48 °C/3 mmHg (lit.)
density
0.951 g/mL at 25 °C (lit.)
functional group
phosphine
SMILES string
CC(C)(C)P(Cl)C(C)(C)C
InChI
1S/C8H18ClP/c1-7(2,3)10(9)8(4,5)6/h1-6H3
InChI key
MCRSZLVSRGTMIH-UHFFFAOYSA-N
General description
Di-tert-butylchlorophosphine belongs to the class of phosphine ligands. It is used for cross-coupling reactions because of the flexibility of its electronic and steric properties. It plays a key role in stabilizing and activating the central metal atom and is used in reactions such as transition metal-catalyzed C-O, C-N, and C-C bond-forming reactions.
Application
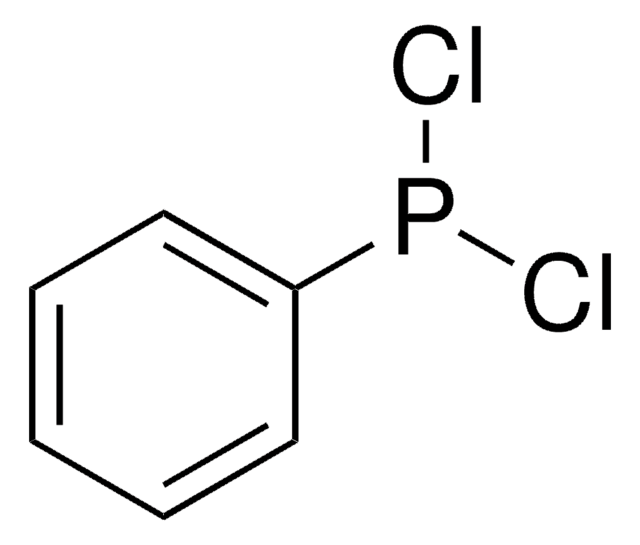
Di-tert-butylchlorophosphine can be used as a ligand in:
- The Pd-catalyzed amination reaction with aryl halides.
- The Pd-catalyzed Suzuki-Miyaura cross-coupling of arylboronic acids with aryl bromides and chlorides.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
143.6 °F - closed cup
Flash Point(C)
62 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Jesudoss V Kingston et al.
The Journal of organic chemistry, 72(8), 2816-2822 (2007-03-24)
Pro-azaphosphatrane 1a [P(iBuNCH2CH2)3N] reacts with iodine under mild conditions to give [IP(iBuNCH2CH2)3N]I in excellent yield, which on subsequent reaction with ammonia followed by deprotonation with KOtBu provided HN=P(iBuNCH2CH2)3N (3a) in quantitative yield. Reaction of 3a with R'2PCl afforded sterically bulky
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service