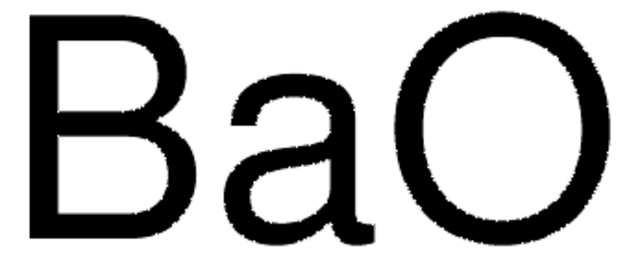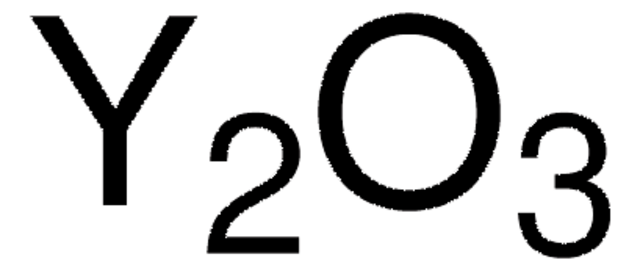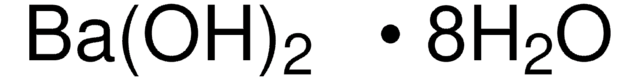All Photos(2)
About This Item
Linear Formula:
BaO
CAS Number:
Molecular Weight:
153.33
EC Number:
MDL number:
UNSPSC Code:
12352303
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Quality Level
Assay
97%
form
powder
reaction suitability
reagent type: catalyst
core: barium
impurities
1-4% adsorbed H2O/CO2
density
5.72 g/mL at 25 °C (lit.)
SMILES string
O=[Ba]
InChI
1S/Ba.O
InChI key
QVQLCTNNEUAWMS-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Barium oxide, also known as barium(II) oxide, is a white or off-white fine powder with a melting point exceeding 1,800 °C. It is a highly stable compound with a high melting point and good chemical resistance, making it suitable for use in a wide range of applications. There are several methods to produce barium oxide including the thermal decomposition of barium carbonate and the electrolysis of barium chloride.
Application
Barium oxide is used in a variety of applications. It is used as a raw material in the production of ceramics, such as glazes and ceramic pigments. It is used as a refractory material in the production of furnace linings and high-temperature applications. It is used as a fluxing agent in the production of glass, helping to lower the melting point and improve the clarity of glass. It is used as a catalyst in the production of polyethylene and other polymers. It is often used as a starting material to produce other barium compounds or dope semiconductors.
Features and Benefits
Used in the synthesis of the high-κ material BST (barium strontium titanate). Used in the study of NOX storage.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Oral - Acute Tox. 4 Inhalation - Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
6.1D - Non-combustible acute toxic Cat.3 / toxic hazardous materials or hazardous materials causing chronic effects
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Sonia Kudłacik-Kramarczyk et al.
Materials (Basel, Switzerland), 13(15) (2020-07-29)
Barium titanate is a ferroelectric perovskite with unique electric properties; therefore, it is widely applied in the fabrication of inorganic coatings or thin films, capacitors, or in the production of devices for energy storage and conversion. This paper describes the
Lei Yang et al.
Nature communications, 2, 357-357 (2011-06-23)
The existing Ni-yttria-stabilized zirconia anodes in solid oxide fuel cells (SOFCs) perform poorly in carbon-containing fuels because of coking and deactivation at desired operating temperatures. Here we report a new anode with nanostructured barium oxide/nickel (BaO/Ni) interfaces for low-cost SOFCs
Paolo Raiteri et al.
Journal of physics. Condensed matter : an Institute of Physics journal, 23(33), 334213-334213 (2011-08-05)
A new reactive force field to describe proton diffusion within the solid oxide fuel cell material BaZrO(3) has been derived. Using a quantum mechanical potential energy surface, the parameters of an interatomic potential model to describe hydroxyl groups within both
Garima Tripathi et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 66(4-5), 1307-1311 (2006-08-22)
Radiative properties of Er3+-doped tertiary bismuth glass has been analyzed by the Judd-Ofelt theory. NIR to visible upconversion in the Er3+-doped glass has been reported. The mechanism for the upconversion is explained on the basis of quadratic dependence on excitation
Shunyi Li et al.
Journal of physics. Condensed matter : an Institute of Physics journal, 23(33), 334202-334202 (2011-08-05)
The electrical properties of (Ba, Sr)TiO(3) (BST) thin films are studied using different combinations of Pt and tin-doped indium oxide (In(2)O(3):Sn, ITO) as electrode material. With Pt as bottom and top electrode the films show insulating behaviour with a low
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 288497-250G | 4061838145659 |
| 288497-50G | 4061826273555 |
| 288497-5G |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service