272000
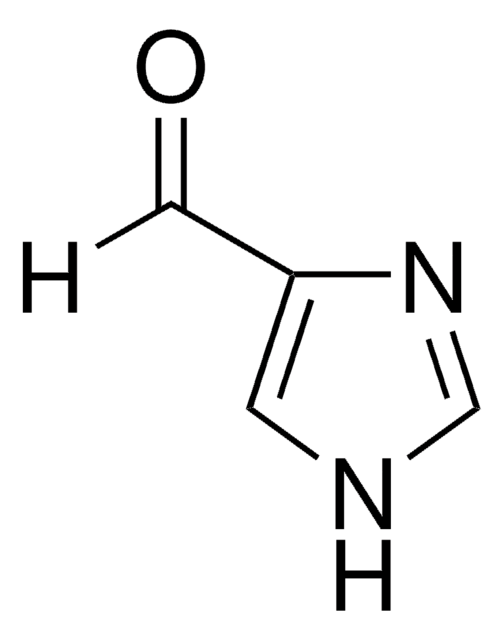
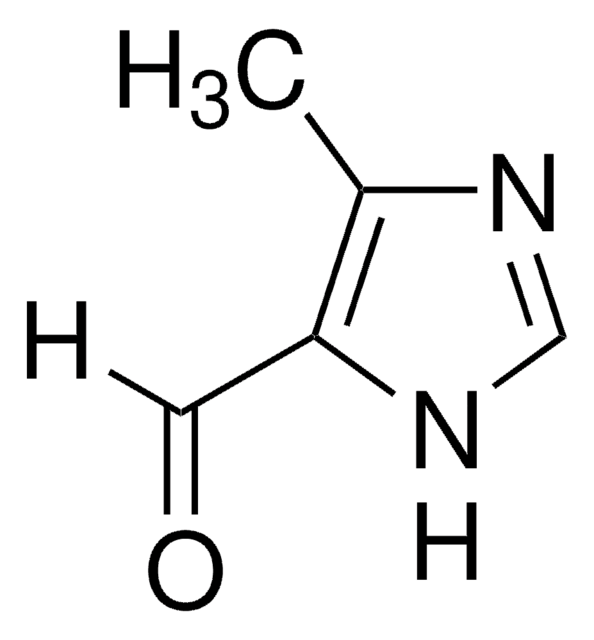
2-Imidazolecarboxaldehyde
97%
Synonym(s):
1H-Imidazole-2-carbaldehyde, 2-Formylimidazole, 2-Imidazolecarbaldehyde, 2-Imidazolylformaldehyde, Imidazole-2-formaldehyde
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C4H4N2O
CAS Number:
Molecular Weight:
96.09
Beilstein:
4371302
MDL number:
UNSPSC Code:
12352005
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
mp
209 °C (dec.) (lit.)
functional group
aldehyde
SMILES string
[H]C(=O)c1ncc[nH]1
InChI
1S/C4H4N2O/c7-3-4-5-1-2-6-4/h1-3H,(H,5,6)
InChI key
XYHKNCXZYYTLRG-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
2-Imidazolecarboxaldehyde was used in the preparation of tridentate Schiff-base carboxylate-containing ligands by undergoing condensation reaction with amino acids β-alanine and 2-aminobenzoic acid. It was also used in a study of the imidazole-directed allylation of aldimines.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
E Colacio et al.
Inorganic chemistry, 39(13), 2882-2890 (2001-03-10)
Tridentate Schiff-base carboxylate-containing ligands, derived from the condensation of 2-imidazolecarboxaldehyde with the amino acids beta-alanine (H2L1) and 2-aminobenzoic acid (H2L5) and the condensation of 2-pyridinecarboxaldehyde with beta-alanine (HL2), D,L-3-aminobutyric acid (HL3), and 4-aminobutyric acid (HL4), react with copper(II) perchlorate to
Rahul Sharma et al.
Environmental toxicology and pharmacology, 80, 103454-103454 (2020-07-10)
The present armamentarium of commercially available antidotes provides limited protection against the neurological effects of organophosphate exposure. Hence, there is an urgent need to design and develop molecules that can protect and reactivate inhibited-AChE in the central nervous system. Some
Nicholas R Perl et al.
Organic letters, 9(18), 3699-3701 (2007-08-10)
A new chiral allylchlorosilane has been developed that allows the highly enantioselective allylation and crotylation of a range of 2-imidazolylaldimines and ketimines. The method may be exploited for the protecting group-free synthesis of a diverse array of imidazole-bearing chiral carbinamines
Ze-Yu Jiang et al.
Angewandte Chemie (International ed. in English), 56(17), 4767-4771 (2017-03-28)
A crack-free sub-nanometer composite structure for the study of ion transfer was constructed by in situ growth of ZIF-90 [Zn(ICA)
Tohid Taghizadeh et al.
International journal of biological macromolecules, 166, 1301-1311 (2020-11-09)
A zeolitic imidazolate framework (ZIF-90) has been synthesized through solvothermal method. The structure was characterized by means of FT-IR spectroscopy, X-ray diffraction, thermogravimetric analysis (TGA), and scanning electron microscopy (SEM)/energy dispersive X-ray spectroscopy (EDS). The synthesized ZIF-90 was applied as
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service