264431
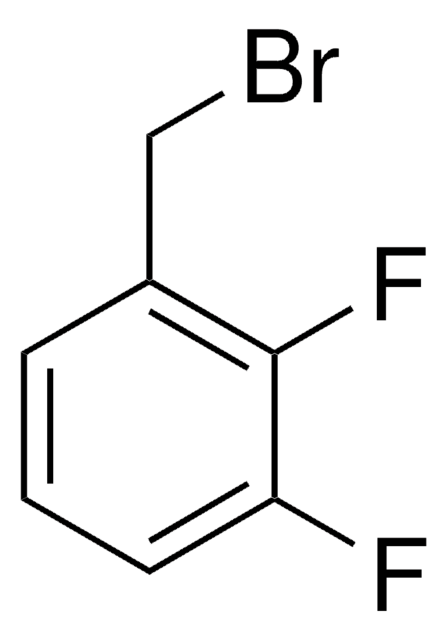
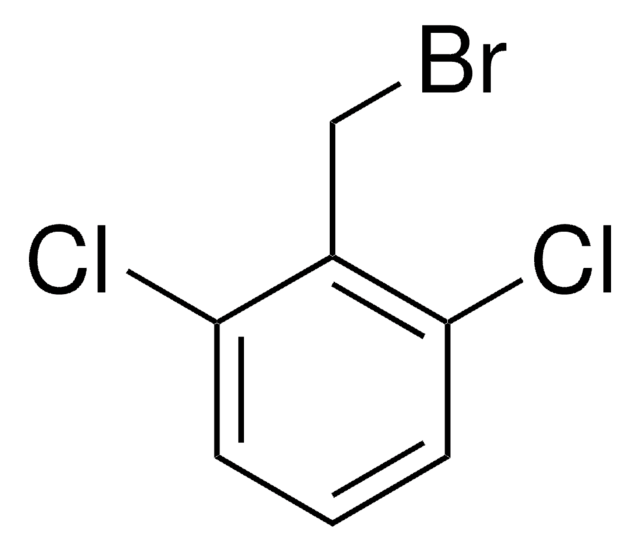
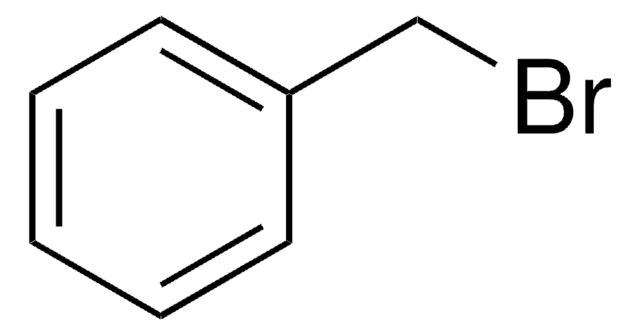
2,6-Difluorobenzyl bromide
97%
Synonym(s):
α-Bromo-2,6-difluorotoluene
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
F2C6H3CH2Br
CAS Number:
Molecular Weight:
207.02
Beilstein:
2083943
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
solid
mp
52-55 °C (lit.)
functional group
bromo
fluoro
SMILES string
Fc1cccc(F)c1CBr
InChI
1S/C7H5BrF2/c8-4-5-6(9)2-1-3-7(5)10/h1-3H,4H2
InChI key
LSXJPJGBWSZHTM-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
2,6-Difluorobenzyl bromide has been used:
- as reagent in alkylation of the quinazoline-2-thioxo-4-one
- in the synthesis of 1,3,5-triazine-2,4,6-triones
- in the preparation of new classes of inhibitors of bovine viral diarrhea virus (as a surrogate virus for hepatitis C virus)
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Zhiqiang Guo et al.
Bioorganic & medicinal chemistry letters, 15(3), 693-698 (2005-01-25)
A convenient one-pot synthetic route was developed for the preparation of asymmetric 1,3-dialkyl-1,3,5-triazine-2,4,6-triones from readily available alkyl- or aryl-isocyanates, primary amines and N-chlorocarbonyl isocyanate in excellent yields. Subsequent alkylation with N-protected amino alcohols afforded the desired 1,3,5-triazine-2,4,6-triones in good yields.
Gerhard Puerstinger et al.
Bioorganic & medicinal chemistry letters, 16(20), 5345-5349 (2006-08-12)
A novel class of inhibitors of pestiviruses (5-substituted 2-phenyl-5H-imidazo[4,5-c]pyridines) is described. Modification of the substituent in position 5 resulted in analogues with high activity (EC(50)<100nM) and selectivity (SI>1000) against the pestivirus BVDV (bovine viral diarrhea virus).
Efficient solid-phase synthesis of quinazoline-2-thioxo-4-ones with SynPhase? lanterns.
Makino S, et al.
Tetrahedron Letters, 41(43), 8333-8337 (2000)
Gwang-Noh Ahn et al.
Lab on a chip, 19(20), 3535-3542 (2019-09-27)
Microreactors are emerging as an efficient, sustainable synthetic tool compared to conventional batch reactors. Here, we present a new numbering-up metal microreactor by integrating a flow distributor and a copper catalytic module for high productivity of a commercial synthetic drug.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service