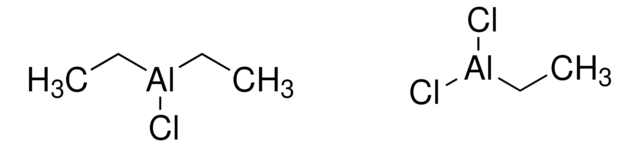252662
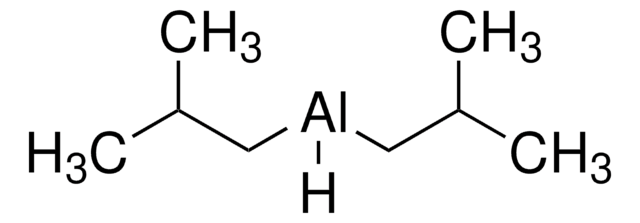
Triethylaluminum solution
1.0 M in hexanes
Synonym(s):
Aluminumtriethanide
About This Item
Recommended Products
form
liquid
Quality Level
concentration
1.0 M in hexanes
density
0.692 g/mL at 25 °C
SMILES string
CC[Al](CC)CC
InChI
1S/3C2H5.Al/c3*1-2;/h3*1H2,2H3;
InChI key
VOITXYVAKOUIBA-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
- As a reagent in Alumino-Mannich reaction, where it reacts with iminium ion substrates for which the corresponding Petasis borono-Mannich reaction is unsuccessful.
- In the synthesis of macrocyclic binuclear aluminum complexes with efficient activities toward ring-opening polymerization of ε-caprolactone.
- To promote Claisen rearrangement of allyl vinyl ether derivatives along with the transfer of ethyl group to aldehydic carbon.
- As a reagent for copper-catalyzed asymmetric conjugate addition of alkenyl- and alkylalanes to α,β-unsaturated lactams.
- As a precursor to synthesize diethylaluminium cyanide, which is used as hydrocyanating reagent.
Signal Word
Danger
Hazard Statements
Hazard Classifications
Aquatic Chronic 2 - Asp. Tox. 1 - Eye Dam. 1 - Flam. Liq. 2 - Pyr. Liq. 1 - Repr. 2 - Self-heat. 2 - Skin Corr. 1A - STOT SE 3 - Water-react 1
Target Organs
Respiratory system
Supplementary Hazards
Storage Class Code
4.2 - Pyrophoric and self-heating hazardous materials
WGK
WGK 3
Flash Point(F)
-0.4 °F - closed cup
Flash Point(C)
-18 °C - closed cup
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service