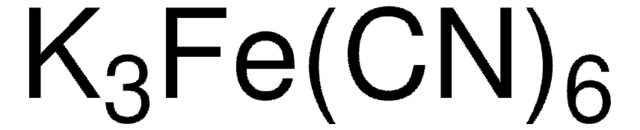209104
Potassium osmate(VI) dihydrate
Synonym(s):
Dipotassium tetrahydroxodioxoosmate, Potassium dioxidodioxoosmium dihydrate, Potassium osmium oxide
About This Item
Recommended Products
form
powder
Quality Level
reaction suitability
reagent type: oxidant
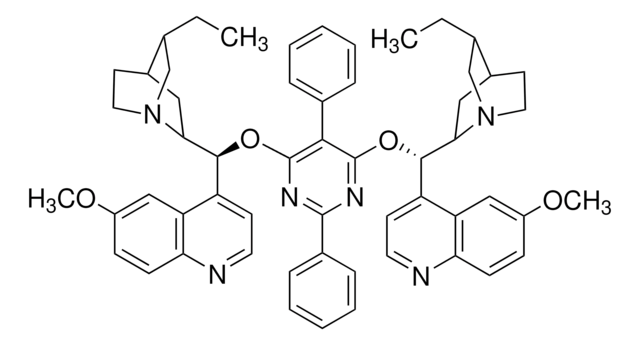
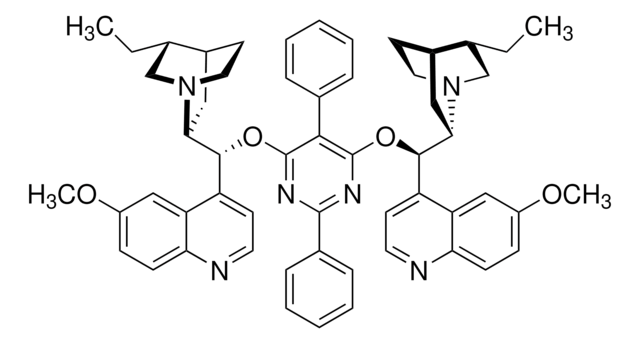
SMILES string
O.O.[K+].[K+].[O-][Os]([O-])(=O)=O
InChI
1S/2K.2H2O.4O.Os/h;;2*1H2;;;;;/q2*+1;;;;;2*-1;
InChI key
DGODWNOPHMXOTR-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- As a reagent for the stereoselective synthesis of 2,3,4-unprotected β-N-Glycopyranosides via palladium-catalyzed Tsuji-Trost amination.
- As a catalyst for asymmetric synthesis of carboranylated diols bearing two adjacent stereocenters.
- To prepare Osmium complexes for voltammetric analysis of polysaccharides.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral
Storage Class Code
6.1B - Non-combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
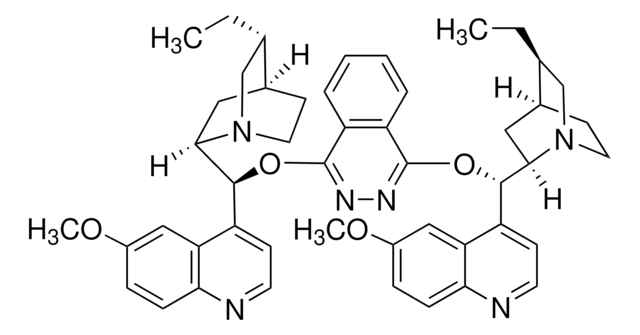
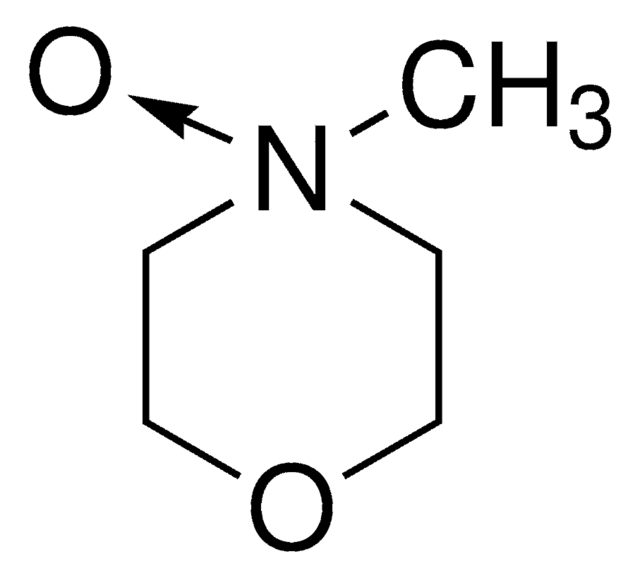
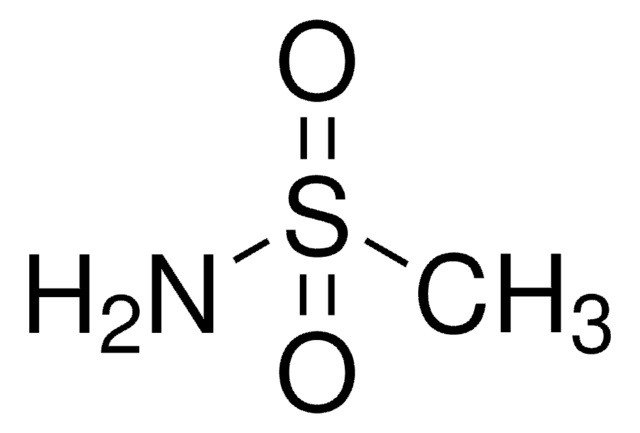
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service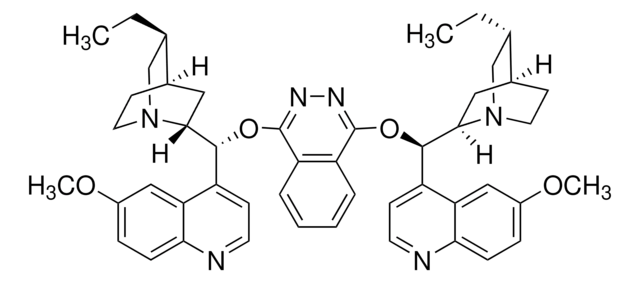


![1,4-Diazabicyclo[2.2.2]octane ReagentPlus®, ≥99%](/deepweb/assets/sigmaaldrich/product/structures/366/129/a6ff4175-974d-4fac-9038-b35e508ef252/640/a6ff4175-974d-4fac-9038-b35e508ef252.png)