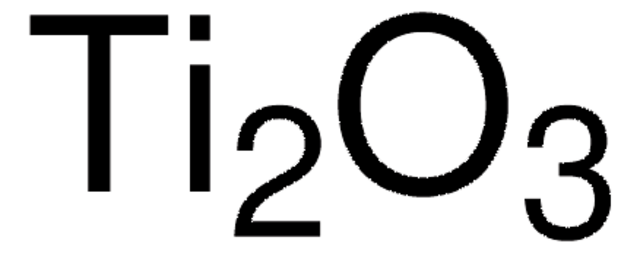204757
Titanium(IV) oxide, rutile
≥99.98% trace metals basis
Synonym(s):
Titanium dioxide
About This Item
Recommended Products
Quality Level
Assay
≥99.98% trace metals basis
form
powder
reaction suitability
reagent type: catalyst
core: titanium
density
4.17 g/mL at 25 °C (lit.)
SMILES string
O=[Ti]=O
InChI
1S/2O.Ti
InChI key
GWEVSGVZZGPLCZ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
Features and Benefits
- Chemical stability
- Non-toxicity
- Excellent stability against photocorrosion
- Ease of further functionalization
Other Notes
Storage Class Code
13 - Non Combustible Solids
WGK
nwg
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Electronically, it behaves as a wide band gap (3.2 eV) semiconductor and exhibits memristor properties.2 Optically, TiO2 has high opacity with a very high refractive index3 (>2.4), and it exhibits strong absorbance in the UV range.
The prevailing strategies for heat and electric-power production that rely on fossil and fission fuels are having a negative impact on the environment and on our living conditions.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service