196967
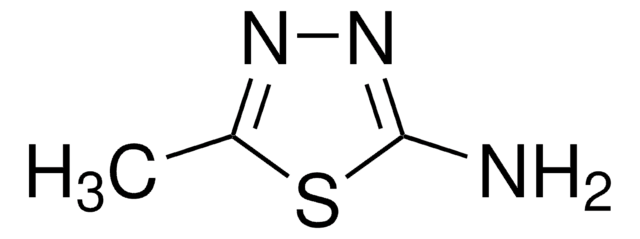
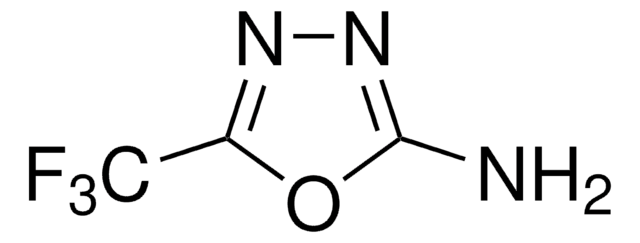
2-Amino-5-trifluoromethyl-1,3,4-thiadiazole
97%
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C3H2F3N3S
CAS Number:
Molecular Weight:
169.13
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
solid
mp
225-227 °C (lit.)
solubility
methanol: soluble 0.25 g/10 mL, clear, colorless
functional group
fluoro
SMILES string
Nc1nnc(s1)C(F)(F)F
InChI
1S/C3H2F3N3S/c4-3(5,6)1-8-9-2(7)10-1/h(H2,7,9)
InChI key
LTEUXHSAYOSFGQ-UHFFFAOYSA-N
Application
2-Amino-5-trifluoromethyl-1,3,4-thiadiazole was used in the synthesis of:
- series of 2-sulfonamido/trifluoromethyl-6-(4′-substituted aryl/heteroaryl)imidazo[2,1-b]-1,3,4-thiadiazole derivatives
- 2-trifluoromethyl/sulfonamido-5,6-diarylsubstituted imidazo[2,1-b]-1,3,4-thiadiazole derivatives
- bicyclic bridgehead itrogen heterocycles
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Andanappa K Gadad et al.
Bioorganic & medicinal chemistry, 12(21), 5651-5659 (2004-10-07)
A series of 2-sulfonamido/trifluoromethyl-6-(4'-substituted aryl/heteroaryl)imidazo[2,1-b]-1,3,4-thiadiazole derivatives (II) have been synthesized by reaction of 2-amino-5-sulfonamido/trifluoromethyl-1,3,4-thiadiazoles and an appropriate alpha-haloaryl/heteroaryl ketones. Further 5-bromo (III), 5-thiocyanato (IV), 5-gaunylhydrazone (V) derivatives were synthesized in order to study the effect of these substituents on biological
Andanappa K Gadad et al.
Bioorganic & medicinal chemistry, 16(1), 276-283 (2007-10-17)
A series of 2-trifluoromethyl/sulfonamido-5,6-diarylsubstituted imidazo[2,1-b]-1,3,4-thiadiazole derivatives 15a-j have been synthesized by the reaction of 2-amino-5-trifluoromethyl/sulfonamido-1,3,4-thiadiazoles 14a-b and appropriately substituted alpha-bromo-1,2-(p-substituted)diaryl-1-ethanones 13a-h. Structures of these compounds were established by IR, (1)H NMR, (13)C NMR, Mass, and HRMS data. The selected compounds
Bridgehead nitrogen heterocycles. II. Formation by reaction of. alpha.-amino nitrogen heterocyclic compounds with chlorothioformyl chloride.
Pilgram K and Skiles RD.
The Journal of Organic Chemistry, 38(8), 1575-1578 (1973)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service