187186
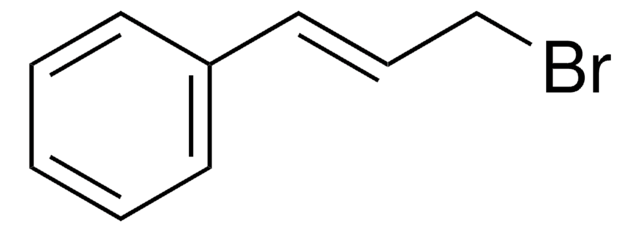
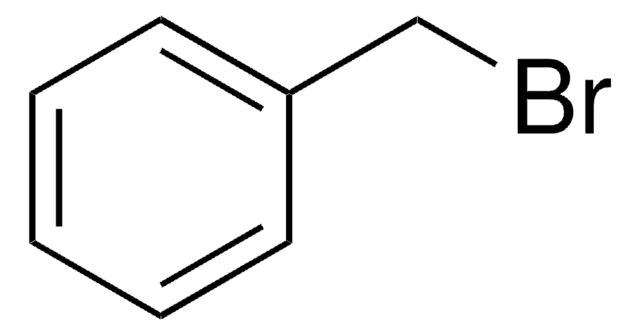
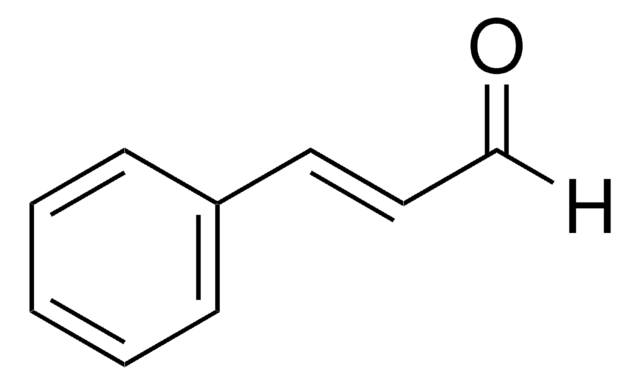
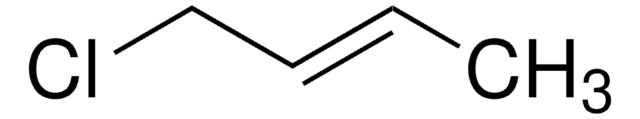
Cinnamyl chloride
95%
Synonym(s):
(3-Chloropropenyl)benzene
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
C6H5CH=CHCH2Cl
CAS Number:
Molecular Weight:
152.62
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
95%
form
liquid
refractive index
n20/D 1.584 (lit.)
bp
108 °C/12 mmHg (lit.)
mp
−19 °C (lit.)
density
1.096 g/mL at 25 °C (lit.)
functional group
chloro
phenyl
storage temp.
2-8°C
SMILES string
ClC\C=C\c1ccccc1
InChI
1S/C9H9Cl/c10-8-4-7-9-5-2-1-3-6-9/h1-7H,8H2/b7-4+
InChI key
IWTYTFSSTWXZFU-QPJJXVBHSA-N
General description
Cinnamyl chloride reacts regioselectively with aryl and alkenylgold(I) phosphanes in the presence of palladium catalyst in THF to afford the α-substitution product†.
Application
Cinnamyl chloride was used in the enantioselective total synthesis of helioporins C and E, bioactive marine diterpenes.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Muta. 2 - Repr. 2 - Skin Irrit. 2 - Skin Sens. 1B - STOT RE 2
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
174.2 °F - closed cup
Flash Point(C)
79 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Wibke Lölsberg et al.
Organic letters, 14(23), 5996-5999 (2012-11-15)
A short and enantioselective total synthesis of helioporins C and E, which are bioactive marine diterpenes containing a serrulatane or amphilectane skeleton, was elaborated. The chirogenic step, i.e. a Cu(I)-catalyzed allylic alkylation of a cinnamyl chloride with methylmagnesium bromide, proceeded
Juan Carlos Rueda et al.
Polymers, 12(6) (2020-06-26)
Stiff thermosensitive hydrogels (HG) were synthesized by self-crosslinking free radical polymerization of N,N-dimethylacrylamide (DMAA) and N-isopropylacrylamide (NIPAAm), adjusting the degree of swelling by carboxylate-containing sodium acrylate (NaAc) or a 2-oxazoline macromonomer (MM). The formation of hydrogels was possible due to
Palladium-catalyzed cross-coupling reactions of organogold(I) phosphanes with allylic electrophiles.
Miguel Peña-López et al.
Organic & biomolecular chemistry, 10(8), 1686-1694 (2012-01-24)
Aryl and alkenylgold(I) phosphanes react regioselectively with allylic electrophiles such as cinnamyl and geranyl halides (bromide, chloride and acetates) under palladium catalysis in THF at 80 °C to afford the α-substitution product with moderate to high yields. When the reaction
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service