183695
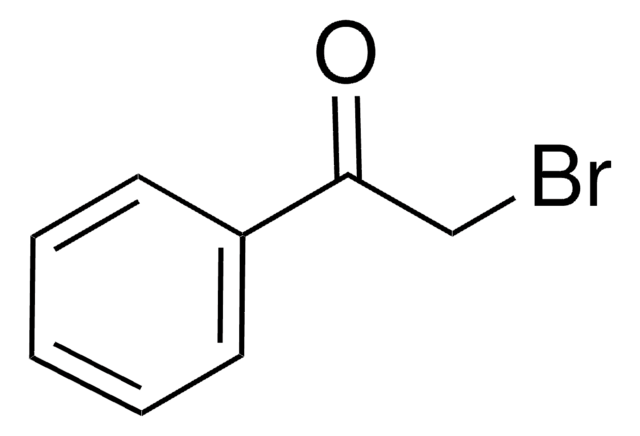
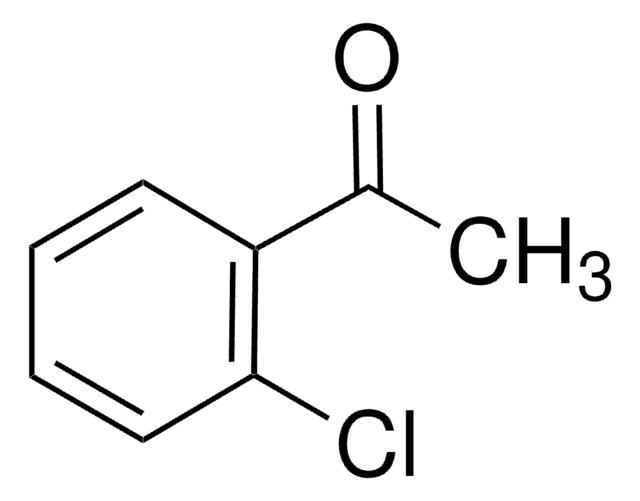
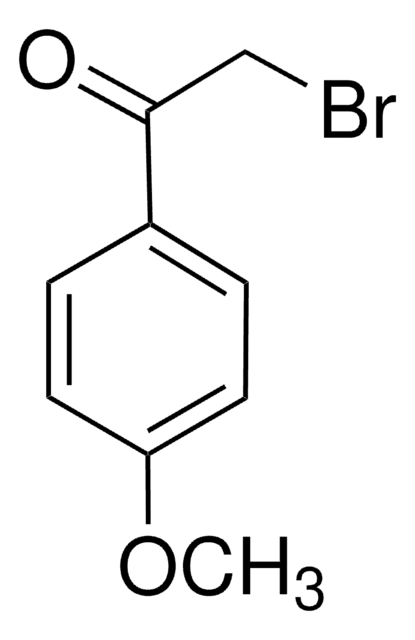
2′-Bromoacetophenone
99%
Synonym(s):
1-Acetyl-2-bromobenzene
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
BrC6H4COCH3
CAS Number:
Molecular Weight:
199.04
Beilstein:
1931534
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
liquid
refractive index
n20/D 1.568 (lit.)
density
1.476 g/mL at 25 °C (lit.)
functional group
bromo
ketone
SMILES string
CC(=O)c1ccccc1Br
InChI
1S/C8H7BrO/c1-6(10)7-4-2-3-5-8(7)9/h2-5H,1H3
InChI key
PIMNFNXBTGPCIL-UHFFFAOYSA-N
General description
2′-Bromoacetophenone (2-Bromoacetophenone) undergoes enantioselective addition reaction with phenylacetylene catalyzed by chiral camphorsulfonamide. It reacts with aliphatic primary amines in the presence of palladium catalyst to afford 3-methyleneisoindolin-1-ones.
Application
2′-Bromoacetophenone (2-Bromoacetophenone) was used in the synthesis of novel series of non-condensed 5,5-bicycles.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
230.0 °F - closed cup
Flash Point(C)
110 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Non-Condensed Trifluoromethylated 5, 5-Bicycles: Synthesis of 2-[3-Alkyl (phenyl)-1H-pyrazol-1-yl]-4-phenyl-5-alkylthiazole and-4, 5, 6, 7-tetrahydrobenzothiazole Systems.
Bonacorso HG, et al.
Synthesis, 2002(08), 1079-1083 (3003)
Enantioselective alkynylation of aromatic ketones catalyzed by chiral camphorsulfonamide ligands.
Gui Lu et al.
Angewandte Chemie (International ed. in English), 42(41), 5057-5058 (2003-11-05)
Palladium-catalysed convenient synthesis of 3-methyleneisoindolin-1-ones.
Cho CS, et al.
Synthetic Communications, 32(!2), 1821-1827 (2002)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service