All Photos(1)
About This Item
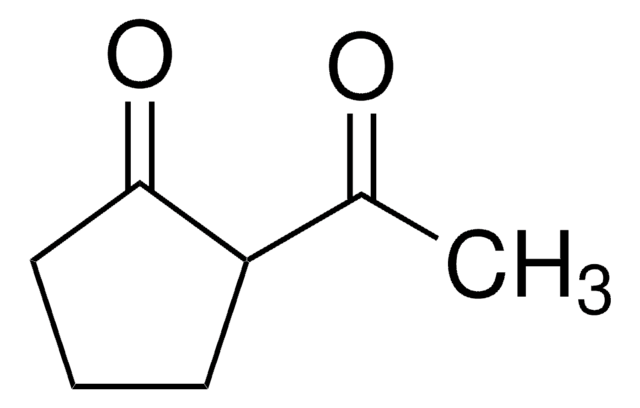
Linear Formula:
CH3COC6H9(=O)
CAS Number:
Molecular Weight:
140.18
Beilstein:
1858621
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
liquid
refractive index
n20/D 1.509 (lit.)
bp
111-112 °C/18 mmHg (lit.)
density
1.078 g/mL at 25 °C (lit.)
functional group
ketone
SMILES string
CC(=O)C1CCCCC1=O
InChI
1S/C8H12O2/c1-6(9)7-4-2-3-5-8(7)10/h7H,2-5H2,1H3
InChI key
OEKATORRSPXJHE-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
The keto-enol tautomerism of 2-acetylcyclohexanone (ACHE) in water was studied.
Application
2-Acetylcyclohexanone was used in the synthesis of anilinoethanolamines.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
174.2 °F - closed cup
Flash Point(C)
79 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Emilia Iglesias
The Journal of organic chemistry, 68(7), 2680-2688 (2003-03-29)
The keto-enol tautomerism of 2-acetylcyclohexanone (ACHE) was studied in water under different experimental conditions. By contrast with other previously studied beta-diketones, the keto-enol interconversion in the ACHE system is a slow process. Under equilibrium conditions, the analysis of the absorbance
Cédric Bouteiller et al.
Organic & biomolecular chemistry, 8(5), 1111-1120 (2010-02-19)
An operationally simple and concise synthesis of anilinoethanolamines, as NMDA NR2B receptor antagonist ifenprodil analogues, was developed via a copper-catalyzed amination of the corresponding bromoarene. Coupling was achieved with linear primary alkylamines, alpha,omega-diamines, hexanolamine and benzophenone imine, as well as
Emilia Iglesias
The Journal of organic chemistry, 68(7), 2689-2697 (2003-03-29)
The kinetic study of the nitrosation of the enol of 2-acetylcyclohexanone (ACHE) has been performed in aqueous acid media in the absence and presence of alpha- and beta-cyclodextrin. The reaction is first-order with respect to both reactants concentration: [nitrite] and
An alternative to the classical α-arylation: the transfer of an intact 2-iodoaryl from ArI(O₂CCF₃)₂.
Zhiyu Jia et al.
Angewandte Chemie (International ed. in English), 53(42), 11298-11301 (2014-09-10)
The α-arylation of carbonyl compounds is generally accomplished under basic conditions, both under metal catalysis and via aryl transfer from the diaryl λ(3)-iodanes. Here, we describe an alternative metal-free α-arylation using ArI(O2CCF3)2 as the source of a 2-iodoaryl group. The
Masaharu Fujita et al.
Journal of applied toxicology : JAT, 39(2), 191-208 (2018-09-18)
The amino acid derivative reactivity assay (ADRA) is an in chemico alternative to animal testing for skin sensitization that solves certain problems found in the use of the direct peptide reactivity assay (DPRA). During a recent validation study conducted at
Articles
Buchwald phosphine ligands for C-C, C-N, and C-O bond formation.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service

![[4,4′-Bis(1,1-dimethylethyl)-2,2′-bipyridine] nickel (II) dichloride](/deepweb/assets/sigmaaldrich/product/structures/471/091/6faa29b1-bf8a-4d87-90b2-4cc55e082620/640/6faa29b1-bf8a-4d87-90b2-4cc55e082620.png)
![(Ir[dF(CF3)ppy]2(dtbpy))PF6](/deepweb/assets/sigmaaldrich/product/structures/982/913/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09/640/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09.png)