All Photos(1)
About This Item
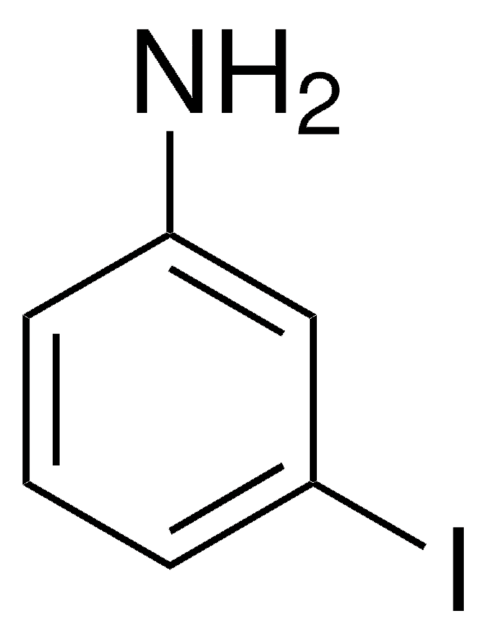
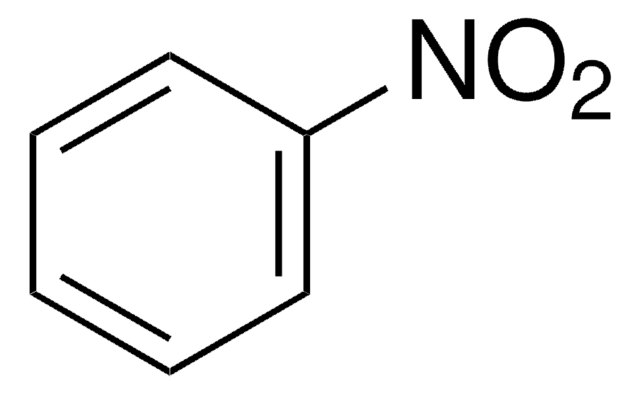
Linear Formula:
IC6H4NO2
CAS Number:
Molecular Weight:
249.01
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
solid
bp
280 °C (lit.)
mp
36-38 °C (lit.)
functional group
iodo
nitro
SMILES string
[O-][N+](=O)c1cccc(I)c1
InChI
1S/C6H4INO2/c7-5-2-1-3-6(4-5)8(9)10/h1-4H
InChI key
CBYAZOKPJYBCHE-UHFFFAOYSA-N
Application
1-Iodo-3-nitrobenzene was used in the synthesis of 5-substituted pyrrolo[3,2-b]pyridine amide and 3′-nitro-4-methylthiobiphenyl. It was used in palladium(0) catalyzed cross-coupling reaction between heteroarylzinc iodide and unsaturated iodide.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Flam. Sol. 1 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
4.1B - Flammable solid hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Preparation and reactions of new zincated nitrogen-containing heterocycles.
Bhanu PAS, et al.
Tetrahedron, 53(21), 7237-7254 (1997)
Hee Jin Kim et al.
Bioorganic & medicinal chemistry letters, 20(1), 413-417 (2009-11-10)
Synthesis of a new series of diarylureas and amides having pyrrolo[3,2-b]pyridine scaffold is described. Their in vitro antiproliferative activity against human melanoma cell line A375 and HS 27 human fibroblast cell line was tested and the effect of substituents on
Rupa Hiremath et al.
Chemical communications (Cambridge, England), (23)(23), 2676-2677 (2004-11-30)
Orthorhombic and triclinic crystals of 2-iodo-4-nitroaniline (INA) grow concomitantly from supersaturated ethanol solutions, but the less stable orthorhombic phase can be selectively grown on 3'-X-4-mercaptobiphenyl (X = NO(2), I) self-assembled monolayer templates.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service