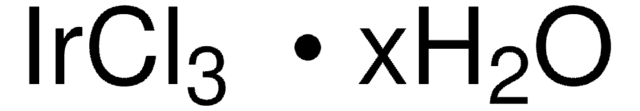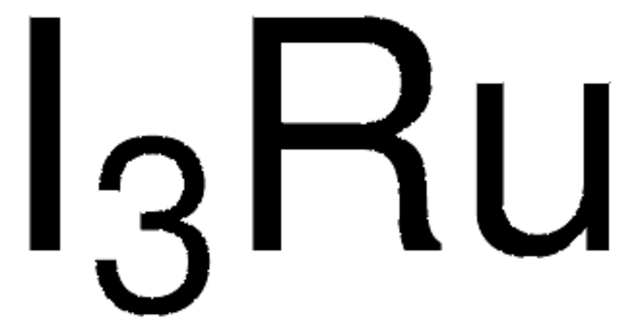10452
Ruthenium(III) chloride trihydrate
technical
Synonym(s):
Ruthenium trichloride trihydrate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
RuCl3 · 3H2O
CAS Number:
Molecular Weight:
261.47
MDL number:
UNSPSC Code:
12161600
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
technical
Quality Level
form
solid
reaction suitability
core: ruthenium
reaction type: C-H Activation
reagent type: catalyst
SMILES string
O.O.O.Cl[Ru](Cl)Cl
InChI
1S/3ClH.3H2O.Ru/h3*1H;3*1H2;/q;;;;;;+3/p-3
InChI key
ZTWIEIFKPFJRLV-UHFFFAOYSA-K
Application
Ruthenium(III) chloride trihydrate can be used as a catalyst:
- Precursor for the hydroamidation of terminalalkynes.
- For the hydrolysis of 4-nitrobenzonitrile to 4-nitrobenzamide in the presence of 1,3,5-triaza-7-phosphaadamantane.
- For the oxidation of thioethers to sulfones in the presence of sodium periodate and acetonitrile.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Jaouhari, R.; Guenot, P.; Dixneuf, P. H.
Chemical Communications (Cambridge, England), 1255-1255 (1986)
Tenaglia, A.; Terranova, E. et al.
Tetrahedron Letters, 32, 1169-1169 (1991)
Sasaki, Y.; Dixneuf, P. H.
The Journal of Organic Chemistry, 52, 314-314 (1987)
Ceria supported ruthenium(0) nanoparticles: Highly efficient catalysts in oxygen evolution reaction.
Elif Demir et al.
Journal of colloid and interface science, 534, 704-710 (2018-10-01)
Ruthenium(0) nanoparticles were successfully prepared on the surface of ceria (Ru0/CeO2) and used as catalysts on glassy carbon electrode (GCE) in oxygen evolution reaction (OER) from water electrolysis at room temperature. Ru0/CeO2 on GCE exhibits high catalytic activity for OER
Synthesis of Orthogonally N-Protected, C-4 Functionalized Cyclic Guanidines from L-Serine
Silva D, et al.
Synlett, 28, 815-818 (2017)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service