SML2022
UCSF924
≥98% (HPLC)
Synonym(s):
6-Methyl-2-((3-phenoxypropylamino)methyl)quinolin-4(1H)-one, ZINC000091726127
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C20H22N2O2
CAS Number:
Molecular Weight:
322.40
UNSPSC Code:
12352200
NACRES:
NA.77
Recommended Products
Assay
≥98% (HPLC)
form
powder
color
white to beige
solubility
DMSO: 2 mg/mL, clear
storage temp.
−20°C
SMILES string
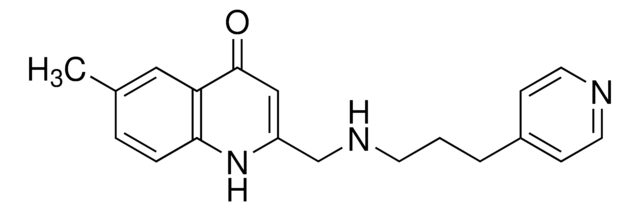
CC1=CC=C(NC(CNCCCOC2=CC=CC=C2)=CC3=O)C3=C1
Biochem/physiol Actions
UCSF924 (Compound 9-6-24; ZINC000091726127) is a selective, high-affinity dopamine D4 receptor (DRD4) partial agonist (Ki = 3 nM for human D4) with a 7.4-fold bias toward arrestin recruitment over Gαi (Gαi/0; Gi/G0) signaling activation with respect to quinpirole. UCSF924 exhibits no detectable affinity for D2, D3 or the F261V/L328F D4 mutant and no agonist activity toward a panel of 320 nonolfactory GPCRs even at a high concentration of 1 μM. The UCSF924 structure analog UCSF924NC (Comound 9-6-16; ZINC000091707446) is the recommended negative control compound with a 1/2500-fold reduced D4 affinity.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Xiaocong M Ye et al.
Bioorganic & medicinal chemistry letters, 23(4), 996-1000 (2013-01-15)
Structure-activity relationship (SAR) of a novel, potent and metabolically stable series of benzo [3.2.1] bicyclic sulfonamide-pyrazoles as γ-secretase inhibitors are described. Compounds that are efficacious in reducing the cortical Aβx-40 levels in FVB mice via oral dose, as well as
Localization and Processing of the Amyloid-β Protein Precursor in Mitochondria-Associated Membranes.
Dolores Del Prete et al.
Journal of Alzheimer's disease : JAD, 55(4), 1549-1570 (2016-12-03)
Alteration of mitochondria-associated membranes (MAMs) has been proposed to contribute to the pathogenesis of Alzheimer's disease (AD). We studied herein the subcellular distribution, the processing, and the protein interactome of the amyloid-β protein precursor (AβPP) and its proteolytic products in
Inger Lauritzen et al.
The Journal of neuroscience : the official journal of the Society for Neuroscience, 32(46), 16243-1655a-16243-1655a (2012-11-16)
Triple-transgenic mice (3xTgAD) overexpressing Swedish-mutated β-amyloid precursor protein (βAPP(swe)), P310L-Tau (Tau(P301L)), and physiological levels of M146V-presenilin-1 (PS1(M146V)) display extracellular amyloid-β peptides (Aβ) deposits and Tau tangles. More disputed is the observation that these mice accumulate intraneuronal Aβ that has been
Rampurna Gullapalli et al.
Drug delivery, 19(5), 239-246 (2012-06-05)
Hydrophilic, non-aqueous solvents are frequently used to solubilize poorly water soluble compounds for use in ALZET® osmotic pumps used during the discovery and preclinical stages. Though these solvents exhibit the potential to solubilize several poorly soluble compounds, the solubilized compounds
Kevin Quinn et al.
Journal of pharmaceutical sciences, 101(4), 1462-1474 (2012-01-04)
ELND006 is a novel gamma secretase inhibitor previously under investigation for the oral treatment of Alzheimer's disease. ELND006 shows poor solubility and has moderate to high permeability, suggesting it is a Biopharmaceutics Classification System Class II compound. The poor absolute
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service