N7502
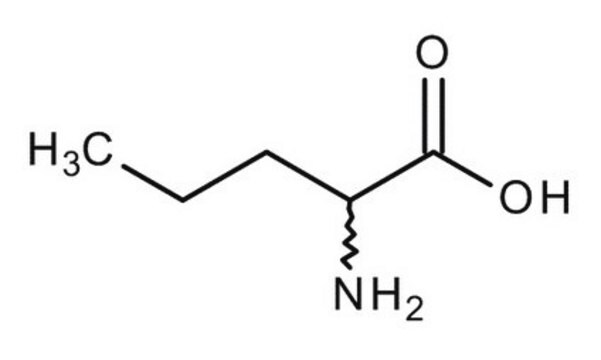
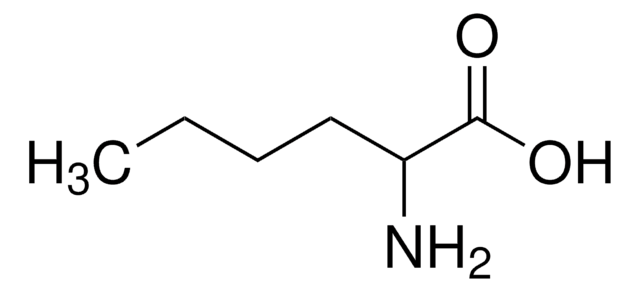
DL-Norvaline
≥98% (HPLC), suitable for HPLC
Synonym(s):
(±)-2-Aminopentanoic acid
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
CH3CH2CH2CH(NH2)COOH
CAS Number:
Molecular Weight:
117.15
Beilstein:
1721163
EC Number:
MDL number:
UNSPSC Code:
12352209
PubChem Substance ID:
NACRES:
NA.26
Recommended Products
Product Name
DL-Norvaline,
Assay
≥98% (HPLC)
Quality Level
form
powder
technique(s)
HPLC: suitable
color
white
mp
>300 °C
SMILES string
CCCC(N)C(O)=O
InChI
1S/C5H11NO2/c1-2-3-4(6)5(7)8/h4H,2-3,6H2,1H3,(H,7,8)
InChI key
SNDPXSYFESPGGJ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Norvaline is a non-proteinogenic α-amino acid and is also called DL-α-aminovaleric acid. It is produced in Gram-negative microorganisms including Escherichia coli and is also a part of the antifungal peptide produced by Bacillus subtilis.
Application
DL-Norvaline has been used as an internal standard:
- to analyze metabolites in cultured mammalian cells by gas chromatography-mass spectrometry (GC-MS)
- to convert cysteine and cystine to Cys MPA (S-2-carboxyethylthio-L-cysteine) and quantify the same using a high-performance liquid chromatography (HPLC) analyzer
- for free amino acid analysis in samples of oocytes by liquid chromatography
Biochem/physiol Actions
DL-Norvaline is a neurotransmitter.
Norvaline mediates physiological and pathophysiological processes promoting nitric oxide production. It is used to treat Alzheimer′s disease and is effective against artificial metabolic syndrome in rats. Norvaline exhibits phase transition upon cooling below 190 K and hence it is useful in molecular machines. DL-Norvaline serves as a prototype for research in polymorphism and molecular dynamics of aliphatic α-amino acids containing linear side-chain. It influences the activity, stability, and side-chain packaging of proteins.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Jens Neu et al.
Physical chemistry chemical physics : PCCP, 20(1), 276-283 (2017-12-06)
dl-Norvaline is a molecular crystal at room temperature and it undergoes a phase transition when cooled below 190 K. This phase transition is believed to be Martensitic, thus making it of particular interest for molecular machines. In this paper we
Esther W Lim et al.
Methods in molecular biology (Clifton, N.J.), 2088, 51-71 (2020-01-02)
Oxidation-reduction (redox) reactions are ubiquitous in biology and typically occur in specific subcellular compartments. In cells, the electron transfer between molecules and organelles is commonly facilitated by pyridine nucleotides such as nicotinamide adenine dinucleotide phosphate (NADPH) and nicotinamide adenine dinucleotide
W Sosroseno et al.
Oral microbiology and immunology, 21(3), 145-150 (2006-04-22)
The aim of the present study was to determine whether or not lipopolysaccharide from Actinobacillus actinomycetemcomitans could stimulate arginase activity in a murine macrophage cell line (RAW264.7 cells). RAW264.7 cells were treated with A. actinomycetemcomitans-lipopolysaccharide or lipopolysaccharide from Escherichia coli
Carl Henrik Görbitz
The journal of physical chemistry. B, 115(10), 2447-2453 (2011-02-22)
The amino acid DL-norvaline undergoes two solid-solid phase transitions between room temperature and -180 °C. Single-crystal X-ray diffraction studies show that the first of these transitions, taking place around -80 °C, is completely reversible with respect to crystal quality, whereas
Ramasamy Sankaranarayanan et al.
Journal of molecular biology, 375(4), 1052-1063 (2007-12-08)
Mycobacterium tuberculosis ornithine carbamoyltransferase (Mtb OTC) catalyzes the sixth step in arginine biosynthesis; it produces citrulline from carbamoyl phosphate (CP) and ornithine (ORN). Here, we report the crystal structures of Mtb OTC in orthorhombic (form I) and hexagonal (form II)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service