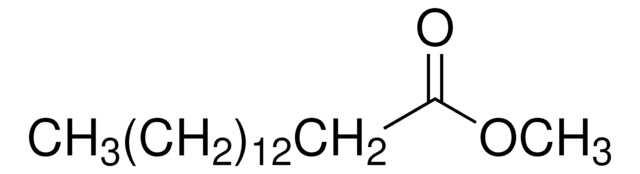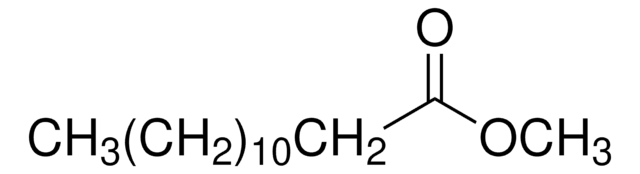M3650
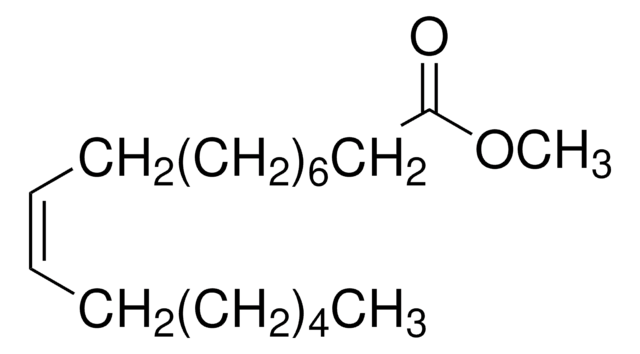
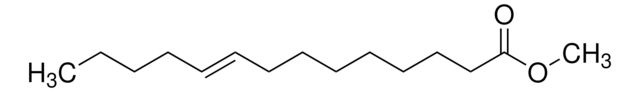
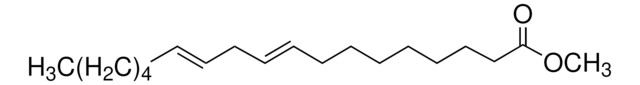
Methyl myristoleate
≥98.5% (capillary GC), liquid
Synonym(s):
Methyl cis-9-tetradecenoate, Myristoleic acid methyl ester
About This Item
Recommended Products
biological source
synthetic (organic)
Quality Level
Assay
≥98.5% (capillary GC)
form
liquid
functional group
ester
lipid type
unsaturated FAs
shipped in
ambient
storage temp.
−20°C
SMILES string
[H]\C(CCCC)=C(/[H])CCCCCCCC(=O)OC
InChI
1S/C15H28O2/c1-3-4-5-6-7-8-9-10-11-12-13-14-15(16)17-2/h6-7H,3-5,8-14H2,1-2H3/b7-6-
InChI key
RWIPSJUSVXDVPB-SREVYHEPSA-N
Looking for similar products? Visit Product Comparison Guide
Packaging
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Protocols
-11-eicosenoate; Methyl elaidate; Methyl linoleate; Methyl myristate; Methyl myristoleate; Methyl palmitate; Methyl palmitoleate; Methyl oleate; Methyl pentadecanoate; Methyl tridecanoate; Methyl behenate; Methyl caprylate; Methyl erucate; Methyl heptadecanoate; Methyl arachidate
GC Analysis of a 37-Component FAME Mix on Omegawax® (15 m x 0.10 mm I.D., 0.10 μm), Fast GC Analysis
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service