F4505
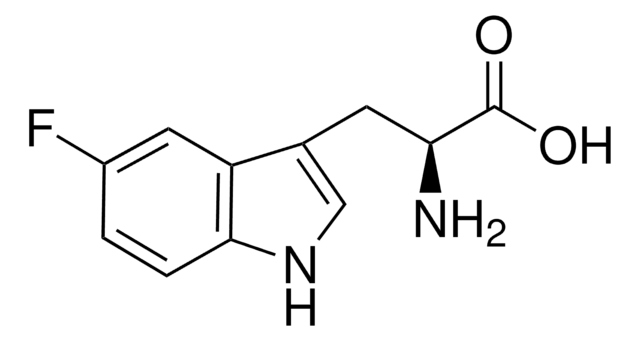
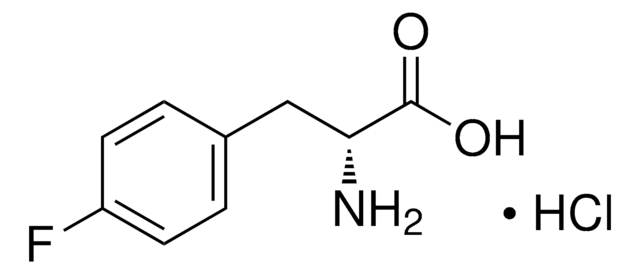
m-Fluoro-DL-tyrosine
≥98%, suitable for ligand binding assays
Synonym(s):
3-fluoro-tyrosine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
FC6H3-4-(OH)CH2CH(NH2)CO2H
CAS Number:
Molecular Weight:
199.18
EC Number:
MDL number:
UNSPSC Code:
12352209
PubChem Substance ID:
NACRES:
NA.26
Recommended Products
Product Name
m-Fluoro-DL-tyrosine,
Assay
≥98%
Quality Level
form
powder
technique(s)
ligand binding assay: suitable
color
white
mp
280 °C (dec.) (lit.)
storage temp.
2-8°C
SMILES string
NC(Cc1ccc(O)c(F)c1)C(O)=O
InChI
1S/C9H10FNO3/c10-6-3-5(1-2-8(6)12)4-7(11)9(13)14/h1-3,7,12H,4,11H2,(H,13,14)
InChI key
VIIAUOZUUGXERI-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Biochem/physiol Actions
M-Fluorotyrosine (m-Fluoro-DL-tyrosine) may be substituted for tyrosine in the biosynthesis of proteins such as β-galactosidases (Escherichia coli), bacteriorhodopsin and intestinal microvillar enzymes, eg. aminopeptidase N, to study the effect of halogenated tryosines on the proteins properties.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
G Xiao et al.
Journal of molecular biology, 281(2), 323-339 (1998-08-12)
The structure of the tetradeca-(3-fluorotyrosyl) M1-1 GSH transferase (3-FTyr GSH transferase), a protein in which tyrosine residues are globally substituted by 3-fluorotyrosines has been determined at 2.2 A resolution. This variant was produced to study the effect on the enzymatic
Mohammad R Seyedsayamdost et al.
Journal of the American Chemical Society, 128(5), 1569-1579 (2006-02-02)
A set of N-acylated, carboxyamide fluorotyrosine (F(n)()Y) analogues [Ac-3-FY-NH(2), Ac-3,5-F(2)Y-NH(2), Ac-2,3-F(2)Y-NH(2), Ac-2,3,5-F(3)Y-NH(2), Ac-2,3,6-F(3)Y-NH(2) and Ac-2,3,5,6-F(4)Y-NH(2)] have been synthesized from their corresponding amino acids to interrogate the detailed reaction mechanism(s) accessible to F(n)()Y*s in small molecules and in proteins. These Ac-F(n)()Y-NH(2)
Prajna P Pal et al.
Biochemistry, 44(10), 3663-3672 (2005-03-09)
Global replacements of tyrosine by 2- and 3-fluorotyrosine in "enhanced green" and "enhanced yellow" mutants of Aequorea victoria green fluorescent proteins (avGFPs) provided protein variants with novel biophysical properties. While crystallographic and modeled structures of these proteins are indistinguishable from
M Ring et al.
Biochemistry and cell biology = Biochimie et biologie cellulaire, 71(3-4), 127-132 (1993-03-01)
An Escherichia coli tyrosine auxotroph (MR1) with an inducible lacZ was generated by mutagenesis. Of several tyrosine derivatives tested, only m-fluorotyrosine supported the growth of this mutant and allowed synthesis of active beta-galactosidase. The pH profiles of the beta-galactosidase that
Idelisa Ayala et al.
Biophysical journal, 89(6), 4171-4179 (2005-09-10)
Incorporation of 3-fluorotyrosine and site-specific mutagenesis has been utilized with Fourier transform infrared (FTIR) spectroscopy and x-ray crystallography to elucidate active-site structure and the role of an active-site residue Tyr34 in human manganese superoxide dismutase (MnSOD). Calculated harmonic frequencies at
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service