49797
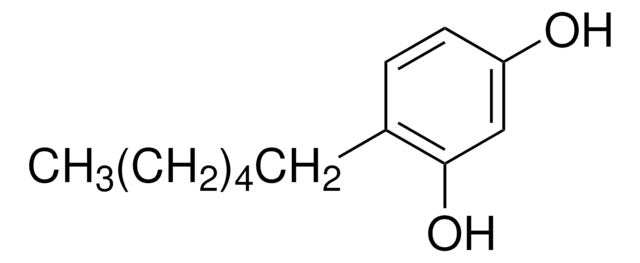
4-Butylresorcinol
analytical standard
Synonym(s):
4-Butyl-1,3-benzenediol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C10H14O2
CAS Number:
Molecular Weight:
166.22
Beilstein:
1942645
MDL number:
UNSPSC Code:
85151701
PubChem Substance ID:
NACRES:
NA.24
Recommended Products
grade
analytical standard
Quality Level
Assay
≥97.0% (GC)
shelf life
limited shelf life, expiry date on the label
application(s)
cleaning products
cosmetics
food and beverages
personal care
format
neat
SMILES string
OC1=C(CCCC)C=CC(O)=C1
InChI
1S/C10H14O2/c1-2-3-4-8-5-6-9(11)7-10(8)12/h5-7,11-12H,2-4H2,1H3
InChI key
CSHZYWUPJWVTMQ-UHFFFAOYSA-N
General description
4-n-butylresorcinol is a derivative of resorcinol and a potent human tyrosinase inhibitor. It may be used to decrease skin irritation and is also known to inhibit melanin production.
Recommended products
Find a digital Reference Material for this product available on our online platform ChemisTwin® for NMR. You can use this digital equivalent on ChemisTwin® for your sample identity confirmation and compound quantification (with digital external standard). An NMR spectrum of this substance can be viewed and an online comparison against your sample can be performed with a few mouseclicks. Learn more here and start your free trial.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Acute 1 - Eye Irrit. 2 - Skin Irrit. 2
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 2
Flash Point(F)
293.0 °F
Flash Point(C)
145 °C
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
The efficacy and safety of 4-n-butylresorcinol 0.1% cream for the treatment of melasma: a randomized controlled split-face trial.
Huh SY, et al.
Annals of Dermatology, 22(1), 21-25 (2010)
William Wargniez et al.
Journal of chromatography. B, Analytical technologies in the biomedical and life sciences, 1060, 416-423 (2017-07-05)
In the present study, three laboratories independently compared percutaneous absorption and distribution of 4-n-butylresorcinol, using human skin from five donors. Each laboratory used the same protocol for percutaneous absorption studies but different LC-MS/MS analytical methods to quantify the test compound.
S-J Lee et al.
International journal of cosmetic science, 39(3), 248-255 (2016-10-27)
4-n-butylresorcinol is a competitive inhibitor of tyrosinase and has been used as an antimelanogenic agent. However, its inhibition mechanism in intact cells is not fully understood. To elucidate the cellular mechanism, we compared in vitro and in vivo inhibitory effects
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service