All Photos(1)
About This Item
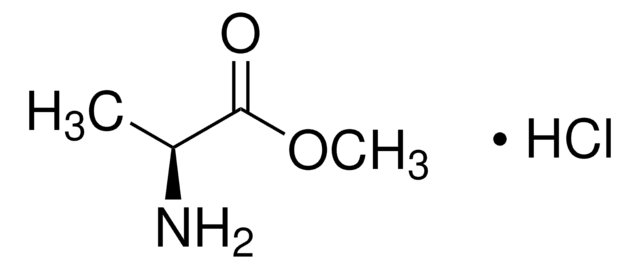
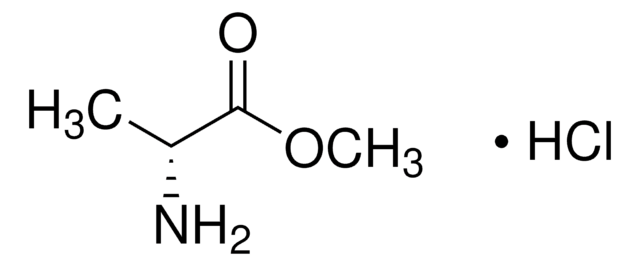
Empirical Formula (Hill Notation):
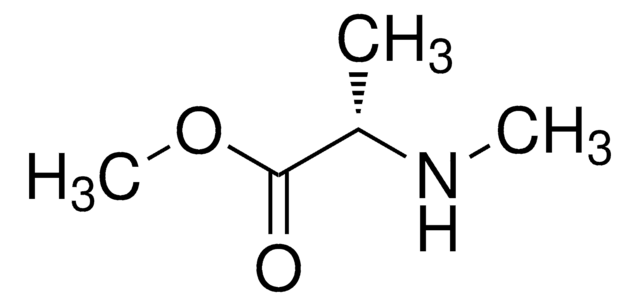
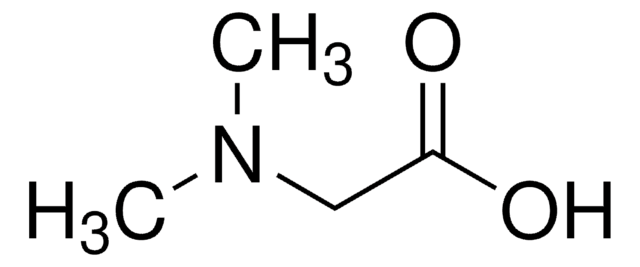
C4H9NO2
CAS Number:
Molecular Weight:
103.12
Beilstein:
1720922
MDL number:
UNSPSC Code:
12352209
eCl@ss:
32160406
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥98.0% (TLC)
form
powder
reaction suitability
reaction type: solution phase peptide synthesis
color
colorless to white
application(s)
peptide synthesis
SMILES string
CN[C@@H](C)C(O)=O
InChI
1S/C4H9NO2/c1-3(5-2)4(6)7/h3,5H,1-2H3,(H,6,7)/t3-/m0/s1
InChI key
GDFAOVXKHJXLEI-VKHMYHEASA-N
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
C H Tan et al.
Biochemical pharmacology, 39(5), 955-958 (1990-03-01)
Synaptosomes isolated from adult rat cerebral cortices were used for studying the uptake of L-leucine by the Na(+)-dependent route. Three non-metabolizable amino acid analogues, which had been used previously to discriminate the Na(+)-dependent A-type uptake system of animal cells, were
G Yadid et al.
Neuroscience, 55(4), 1147-1152 (1993-08-01)
[3H]Glycine is actively taken up into bovine isolated adrenal medulla chromaffin cells with the subsequent catecholamine release. [3H]Glycine uptake has two interaction sites based on relative Km measurements. These sites are inherently distinct since the effects of strychnine and temperature
Y Nishina et al.
Journal of biochemistry, 99(2), 329-337 (1986-02-01)
Resonance Raman (RR) spectra were obtained for the purple complexes of D-amino acid oxidase (DAO) with D-lysine or N-methylalanine. RR spectra of a complex of oxidized DAO with the oxidation product of D-lysine or D-proline were also measured. The isotope
Zarixia Zavala-Ruiz et al.
The Journal of biological chemistry, 278(45), 44904-44912 (2003-09-04)
Crystal structures of the class II major histocompatibilty complex (MHC) protein, HLA-DR1, generally show a tight fit between MHC and bound peptide except in the P6/P7 region of the peptide-binding site. In this region, there is a shallow water-filled pocket
Z Grzonka et al.
Journal of medicinal chemistry, 26(4), 555-559 (1983-04-01)
Eight analogues of oxytocin and arginine-vasopressin were synthesized, in which the proline residue in position 7 was replaced by either sarcosine or N-methylalanine; some of the pharmacological properties of these analogues were evaluated. In peptides containing a beta-mercaptopropionic acid residue
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service