PICM01250
Millicell® Standing Cell Culture Inserts
pore size 0.4 μm, diam. 12 mm, transparent PTFE membrane, hydrophilic, size 24 wells, sterile
Synonym(s):
Millicell Cell Culture Insert, 12 mm, hydrophilic PTFE, 0.4 µm, Millicell-CM, cell culture inserts, permeable culture insert, plate inserts, tissue culture insert, tissue culture plate
About This Item
Recommended Products
material
polystyrene housing
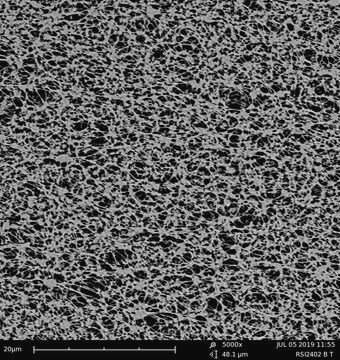
transparent PTFE membrane
Quality Level
sterility
ethylene oxide treated
sterile
feature
hydrophilic
packaging
pack of 50
manufacturer/tradename
Millicell®
parameter
50 °C max. temp.
technique(s)
cell attachment: suitable
cell culture | mammalian: suitable
cell differentiation: suitable
H
10.5 mm
diam.
12 mm
filtration area
0.6 cm2
size
24 wells
surface area
0.6 cm2
working volume
0.6 mL
color
transparent, when wetted
matrix
Biopore™
pore size
0.4 μm
binding type
low binding surface
detection method
fluorometric
shipped in
ambient
General description
With Millicell® inserts, attachment or suspension cells can access media from both their apical and basolateral sides. Cell growth, structure, and function more closely mimic what occurs in vivo. In addition, Millicell® inserts make it possible to study both sides of the cell monolayer.
Millicell® Standing Inserts:
- Promotes excellent cell growth and provides an exceptional opportunity for cell studies
Membrane Type:
Biopore Membrane (hydrophilic PTFE)
- For low protein-binding, live cell viewing, and immunofluorescent applications
Applications
Cell Attachment, Cell Growth, Cell Differentiation , Immunocytochemistry
Application
Cell Culture
Packaging
Legal Information
Storage Class Code
10-13 - German Storage Class 10 to 13
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Protocols
This is a Toluidine Blue Staining protocol listing materials and methods.
This protocol covers three methods for the microscopic examination of cell samples.
This page covers the basic indirect co-culture procedure on both sides of Millicell cell culture insert membranes.
This technical article covers the indirect co-culture of embryonic stem cells with embryonic fibroblasts.
Related Content
This page covers the ECM coating protocols developed for four types of ECMs on Millicell®-CM inserts, Collagen Type 1, Fibronectin, Laminin, and Matrigel.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service