516620
Peroxynitrite
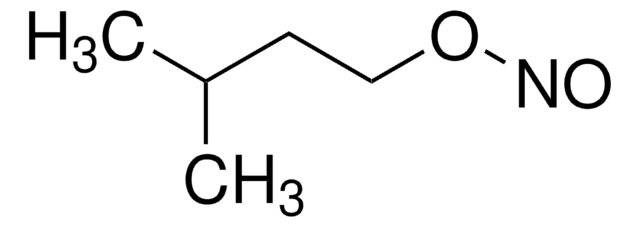
Synthesized from isoamyl nitrite and hydrogen peroxide.
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
UNSPSC Code:
12352200
Recommended Products
Quality Level
form
liquid
manufacturer/tradename
Calbiochem®
storage condition
OK to freeze
avoid repeated freeze/thaw cycles
protect from light
color
yellow
shipped in
wet ice
storage temp.
−70°C
General description
Note: 1 set = 2 x 1 ml.
Synthesized from isoamyl nitrite and hydrogen peroxide. Peroxynitrite (ONOO–) is a strong biological oxidizing agent that reacts with DNA, membrane phospholipids, sulfhydryl groups, and tyrosine. It has been implicated in a variety of patho-physiological conditions including acute respiratory distress syndrome (ARDS), arthritis, atherosclerosis, inflammatory bowel disease, ischemia-reperfusion, N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-mediated neurotoxicity, and septic shock. This product decomposes or isomerizes at pH 8 or less; the half-life at pH 7.5 is ~1.5 s. ε302 nm = 1670M-1cm-1 in 0.1 MNaOH.
Synthesized from isoamyl nitrite and hydrogen peroxide. Peroxynitrite (ONOO–) is a cell-permeable strong biological oxidizing agent that reacts with DNA, membrane phospholipids, sulfhydryl groups, and tyrosine. Has been implicated in a variety of pathophysiological conditions including acute respiratory distress syndrome (ARDS), arthritis, atherosclerosis, inflammatory bowel disease, ischemia-reperfusion, N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-mediated neurotoxicity, and septic shock.
Biochem/physiol Actions
Cell permeable: yes
Primary Target
Oxidizing agent that reacts with DNA, membrane phospholipids, sulfhydryl groups, and tyrosine
Oxidizing agent that reacts with DNA, membrane phospholipids, sulfhydryl groups, and tyrosine
Product does not compete with ATP.
Reversible: no
Packaging
Packaged under inert gas
Warning
Toxicity: Oxidizer (K)
Physical form
Supplied in 4.7% NaOH at 160-200 mM.
Preparation Note
Peroxynitrite is soluble in aqueous buffers that have been pre-purged with an inert gas and pre-cooled to 4°C.
Reconstitution
Protect from heat, light, metal ions, and air. Peroxynitrite is unstable; thaw rapidly, dispense into aliquots, purge tubes with an inert gas, and freeze -70°C. Activity decreases ~2% /day at -20°C.
Other Notes
Due to the nature of the Hazardous Materials in this shipment, additional shipping charges may be applied to your order. Certain sizes may be exempt from the additional hazardous materials shipping charges. Please contact your local sales office for more information regarding these charges.
Marla, S.S., et al. 1997. Proc. Natl. Acad. Sci. USA94, 14243.
Sakuma, S., et al. 1997. Biochem. Biophys. Res. Commun. 230, 476.
Lymar, S.V., et al. 1996. Biochemistry 35, 7855.
Uppu, R.M., and Pryor, W.A. 1996. Methods Enzymol. 269, 322.
Ischiropoulos, H., and al-Medhi, A.B. 1995. FEBS Lett. 364, 279.
Sakuma, S., et al. 1997. Biochem. Biophys. Res. Commun. 230, 476.
Lymar, S.V., et al. 1996. Biochemistry 35, 7855.
Uppu, R.M., and Pryor, W.A. 1996. Methods Enzymol. 269, 322.
Ischiropoulos, H., and al-Medhi, A.B. 1995. FEBS Lett. 364, 279.
Legal Information
CALBIOCHEM is a registered trademark of Merck KGaA, Darmstadt, Germany
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Carc. 2 - Eye Dam. 1 - Met. Corr. 1 - Muta. 2 - Skin Corr. 1B
Storage Class Code
5.1B - Oxidizing hazardous materials
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service