T69000
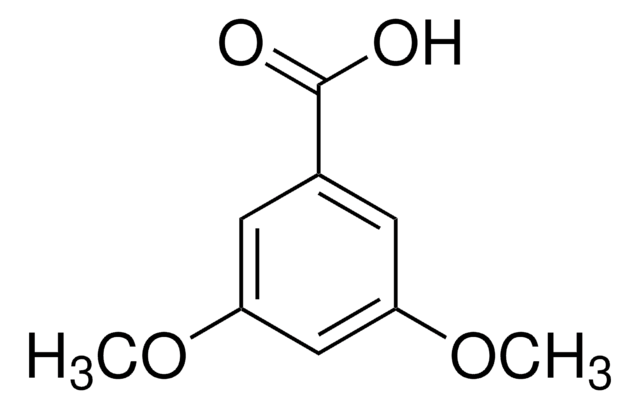
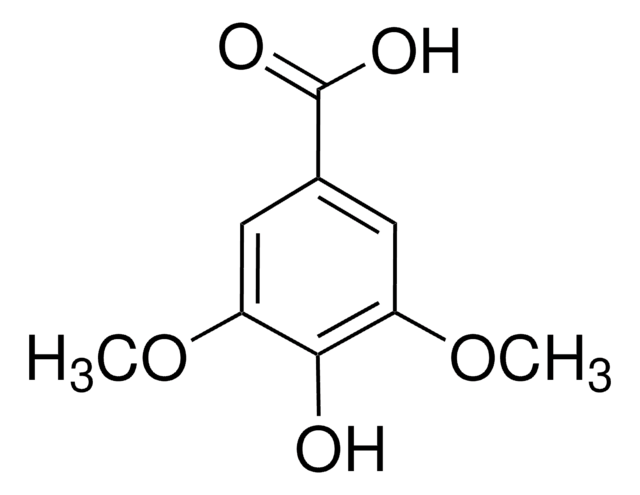
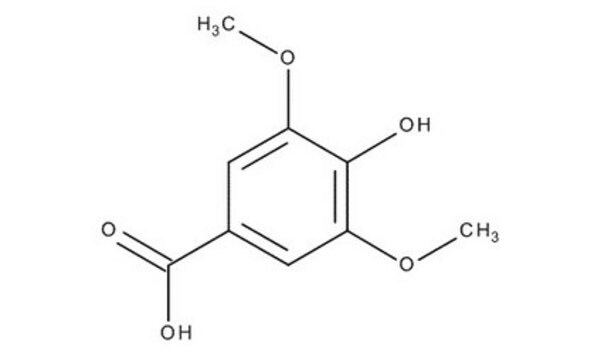
3,4,5-Trimethoxybenzoic acid
ReagentPlus®, 99%
Synonym(s):
Gallic acid trimethyl ether, Trimethylgallic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(CH3O)3C6H2CO2H
CAS Number:
Molecular Weight:
212.20
Beilstein:
884655
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
product line
ReagentPlus®
Assay
99%
bp
225-227 °C/10 mmHg (lit.)
mp
168-171 °C (lit.)
SMILES string
COc1cc(cc(OC)c1OC)C(O)=O
InChI
1S/C10H12O5/c1-13-7-4-6(10(11)12)5-8(14-2)9(7)15-3/h4-5H,1-3H3,(H,11,12)
InChI key
SJSOFNCYXJUNBT-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Legal Information
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Yan-Jun Hu et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 65(3-4), 988-992 (2006-05-09)
The interaction between 3,4,5-trimethoxybenzoic acid (TMBA) and bovine serum albumin (BSA) was studied by fluorescence and UV-vis absorption spectroscopy. In the mechanism discussion, it was proved that the fluorescence quenching of BSA by TMBA is a result of the formation
Y Mimaki et al.
Bioscience, biotechnology, and biochemistry, 60(6), 1049-1050 (1996-06-01)
Bioassay-guided fractionation of the MeOH extract of Ornithogalum saundersiae bulbs led to the isolation of a new cholestane bisdesmoside with potent cytotoxic activities toward leukemia HL-60 and MOLT-4 cells. The structure was deduced mainly from spectroscopic information.
[Synthesis and the study of the effect of new 3,4,5-trimethoxybenzoic acid derivatives on the central nervous system].
R Glinka et al.
Acta poloniae pharmaceutica, 42(2), 117-122 (1985-01-01)
S Kleis-San Francisco et al.
The Journal of experimental zoology, 244(1), 133-143 (1987-10-01)
In the amphibian ovarian follicle, progesterone production is thought to induce maturation of the enclosed oocyte. Intracellular mechanisms regulating these events in the somatic and germ cells are incompletely understood. However, calcium appears to play a role in the production
E Pogonowska-Wala
Polish journal of pharmacology and pharmacy, 33(3), 365-371 (1981-10-01)
Craviten hydrolysis in vitro was studied in human, rat and canine plasma and erythrocytes. Craviten was degraded during the incubation with plasma and the hydrolysis was inhibited by trimethoxybenzoic acid, a metabolite of Craviten. Erythrocyte enzymes do not participate in
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service