P14831
Phenothiazine
≥98%
Synonym(s):
Dibenzo-1,4-thiazine, Thiodiphenylamine, 10H-Phenothiazine
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C12H9NS
CAS Number:
Molecular Weight:
199.27
Beilstein:
143237
EC Number:
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Assay
≥98%
bp
371 °C (lit.)
mp
182-187 °C (lit.)
SMILES string
N1c2ccccc2Sc3ccccc13
InChI
1S/C12H9NS/c1-3-7-11-9(5-1)13-10-6-2-4-8-12(10)14-11/h1-8,13H
InChI key
WJFKNYWRSNBZNX-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Phenothiazine is a versatile building block that can be easily functionalized as it possesses multiple modification sites. Its unique butterfly configuration and electron-rich character are beneficial for its potential use in optoelectronic applications. As it can undergo fast and reversible electron transfer reactions, it is widely used as photoactive material in organic light-emitting devices and solar cells.
Application
Phenothiazine can be used in the following applications:
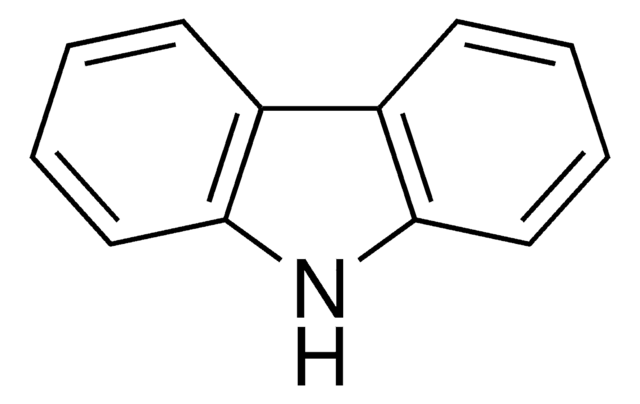
- It can be connected to carbazole by C-N linkage to increase the hole mobility and thermal stability in organic light-emitting devices.
- Used in the fabrication of CMA-based (Carbene-Metal-Amide) white light-emitting OLED.
- It is used as a starting material in the synthesis of dialdehyde monomer. A conjugated copolymer is prepared using this dialdehyde monomer, which is applicable as an emitting layer in PLED.
- Used as a building block in the synthesis of conjugated dendrimers, for the development of light-emitting devices.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Skin Sens. 1 - STOT RE 2 Oral
Target Organs
Blood
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Indian J. Chem. B, 33, 397-397 (1994)
Alexander S Romanov et al.
Chemical science, 11(2), 435-446 (2020-03-20)
Conformationally flexible "Carbene-Metal-Amide" (CMA) complexes of copper and gold have been developed based on a combination of sterically hindered cyclic (alkyl)(amino)carbene (CAAC) and 6- and 7-ring heterocyclic amide ligands. These complexes show photoemissions across the visible spectrum with PL quantum
Synthesis of p-type conjugated dendrimers bearing phenothiazine moiety at the periphery and their light-emitting device characterization
Dong Hoon Choi, et al.
Synthetic Metals, 157(8), 332-335 (2007)
Structure-induced optoelectronic properties of phenothiazine-based materials
Revoju, et al.
Journal of Material Chemistry C, 8(44), 15486-15506 (2020)
Rajendra Kumar Konidena et al.
The Journal of organic chemistry, 80(11), 5812-5823 (2015-05-08)
A series of thienylphenothiazine decorated carbazoles were synthesized and characterized by optical, electrochemical, thermal, and theoretical investigations. Absorption spectra of the compounds are influenced by the substitution pattern and chromophore number density. Compounds containing 2,7-substitution exhibited red-shifted absorption, while the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service

![5H-Dibenz[b,f]azepine 97%](/deepweb/assets/sigmaaldrich/product/structures/396/216/18f00414-a76e-46d7-90cf-820ad902e559/640/18f00414-a76e-46d7-90cf-820ad902e559.png)



![10,11-Dihydro-5H-dibenz[b,f]azepine 97%](/deepweb/assets/sigmaaldrich/product/structures/282/468/27ed6f23-3d01-4628-8293-f0051a6f3b7c/640/27ed6f23-3d01-4628-8293-f0051a6f3b7c.png)