N10845
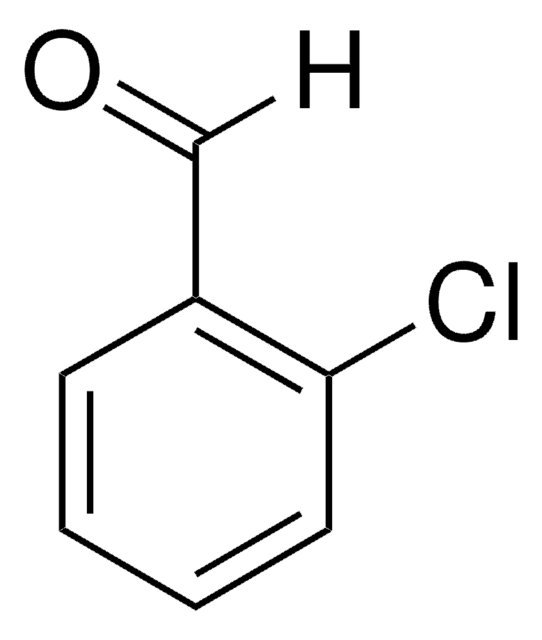
3-Nitrobenzaldehyde
ReagentPlus®, 99%
Synonym(s):
3-Nitrobenzaldehyde, 3-Nitrobenzenecarboxaldehyde, 3-Nitrophenylcarboxaldehyde, 5-Nitrobenzaldehyde, m-Nitrobenzaldehyde
Sign Into View Organizational & Contract Pricing
All Photos(4)
About This Item
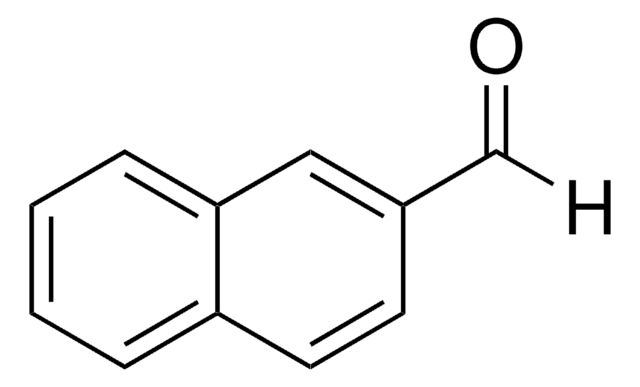
Linear Formula:
O2NC6H4CHO
CAS Number:
Molecular Weight:
151.12
Beilstein:
386795
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
product line
ReagentPlus®
Assay
99%
form
crystals
mp
55-58 °C (lit.)
SMILES string
[O-][N+](=O)c1cccc(C=O)c1
InChI
1S/C7H5NO3/c9-5-6-2-1-3-7(4-6)8(10)11/h1-5H
InChI key
ZETIVVHRRQLWFW-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Legal Information
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Chronic 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
V Karunakaran et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 98, 229-239 (2012-09-11)
FT-IR (4000-400 cm(-1)) and FT-Raman (3500-100 cm(-1)) spectral measurements of 4-chloro-3-nitrobenzaldehyde have been done. Ab initio (HF/6-311+G(d,p)) and DFT (B3LYP/6-311+G(d,p)) calculations have been performed giving energies, optimized structures, harmonic vibrational frequencies, infrared intensities and Raman activities. A detailed interpretation of
E V Sargent et al.
Mutation research, 175(3), 133-137 (1986-11-01)
m-Nitrobenzaldehyde (MNB) was evaluated for mutagenic activity using the Ames microbial mutagenicity test and for its ability to induce DNA single-strand breaks in rat hepatocytes as measured by alkaline elution. MNB was tested in S. typhimurium strains TA1535, TA1537, TA1538
Halil Gökce et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 79(5), 1783-1793 (2011-06-21)
The molecular geometry, vibrational frequencies, 1H and 13C NMR chemical shifts, UV-vis spectra, HOMO-LUMO analyses, molecular electrostatic potentials (MEPs), , thermodynamic properties and atomic charges of 3- and 4-Nitrobenzaldehyde oxime (C7H6N2O3) molecules have been investigated by using Hartree-Fock (HF) and
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service