G6600
Glycine methyl ester hydrochloride
99%, for peptide synthesis
Synonym(s):
Glycine methyl ester·HCl, Methyl glycinate hydrochloride
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
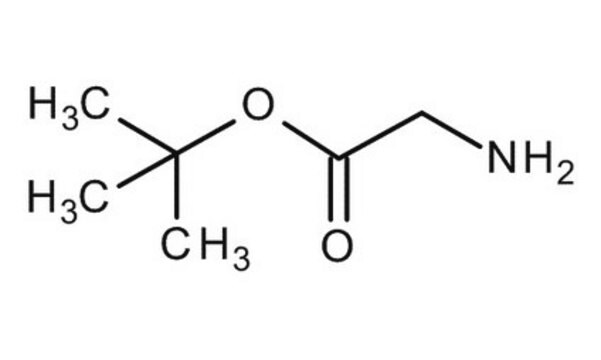
Linear Formula:
NH2CH2COOCH3 · HCl
CAS Number:
Molecular Weight:
125.55
Beilstein:
3593644
EC Number:
MDL number:
UNSPSC Code:
12352209
eCl@ss:
32160406
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
product name
Glycine methyl ester hydrochloride, 99%
Quality Level
Assay
99%
form
powder, crystals or chunks
reaction suitability
reaction type: solution phase peptide synthesis
color
white
mp
175 °C (dec.) (lit.)
application(s)
peptide synthesis
SMILES string
Cl.COC(=O)CN
InChI
1S/C3H7NO2.ClH/c1-6-3(5)2-4;/h2,4H2,1H3;1H
InChI key
COQRGFWWJBEXRC-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Glycine methyl ester hydrochloride, also known as methyl glycinate hydrochloride, is a derivative of glycine, used in the preparation of amino acids and organic compounds.
Application
Glycine methyl ester hydrochloride is used to synthesize cyclophosphazene compounds with amino acid esters as side groups. Additionally, it can be utilized to synthesize peptides in aqueous medium, which makes it a green method for peptide formation.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Akihiko Tohri et al.
European journal of biochemistry, 271(5), 962-971 (2004-03-11)
To elucidate the domains on the extrinsic 23 kDa protein involved in electrostatic interaction with the extrinsic 33 kDa protein in spinach photosystem II, we modified amino or carboxyl groups of the 23 kDa protein to uncharged methyl ester groups
Wenzhong Gao et al.
Organic letters, 7(19), 4241-4244 (2005-09-09)
[reaction: see text] A novel Cu(I)-catalyzed asymmetric 1,3-dipolar cycloaddition of azomethine ylides with acrylates has been developed. Up to 98/2 exo/endo selectivity and up to 98% enantiomeric excess have been achieved.
Glycine enolates: the large effect of iminium ion formation on alpha-amino carbon acidity.
A Rios et al.
Journal of the American Chemical Society, 123(32), 7949-7950 (2001-08-09)
L P Roguin et al.
The Biochemical journal, 224(2), 535-540 (1984-12-01)
Bovine somatotropin with an increasing number of its carboxylate groups modified by reaction with glycine methyl ester in the presence of a water-soluble carbodi-imide was tested for its activity in different bioassays. Only those derivatives which were known to be
J M Delfino et al.
International journal of peptide and protein research, 21(4), 440-450 (1983-04-01)
The reactivity of the carboxyl groups in bovine growth hormone was studied by reaction with 1-ethyl-3(3-dimethylaminopropyl) carbodiimide in the presence of an excess of glycinemethylester. Localization in the molecule of the various kinetically distinguishable carboxyl groups was achieved. Highest reactivity
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service