901635
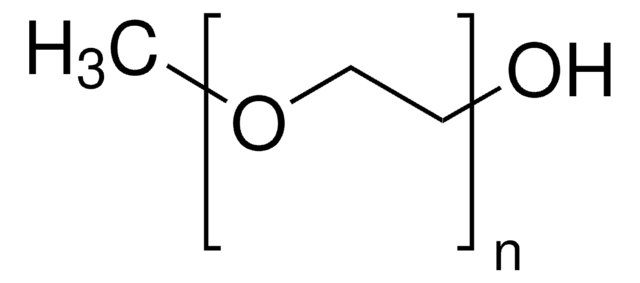
Poly(ethylene glycol) bis(2-pyridyl KAT)
PEG average Mn 10,000
Synonym(s):
KAT PEG, bis KAT PEG, di KAT PEG
About This Item
Recommended Products
form
powder or solid
mol wt
PEG average Mn 10,000
PEG ~10,000 Da
color
off-white to pale yellow
storage temp.
2-8°C
General description
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Related Content
The Bode Group aims to develop new reactions and reagents for the synthesis of complex molecules. The Bode Group has developed N-mesityl-substituted NHCs as organocatalysts for the catalytic generation of reactive species including activated carboxylates, homoenolates, and enolates. These novel catalysts and reactions have made possible a new generation of highly enantioselective annulations from simple starting materials under mild reaction conditions, usually at room temperature and without added reagents. Furthering the goal of designing new reagents to enable the assembly of complex molecules, the Bode group has developed SnAP reagents for the facile, one-pot conversion of aldehydes into N-unprotected, saturated N-heterocycles, including bicyclic and spirocyclic structures. These easy to handle reagents provide a simple and robust alternative to the challenging and restrictive cross-coupling methods for the functionalization of saturated N-heterocycles.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service