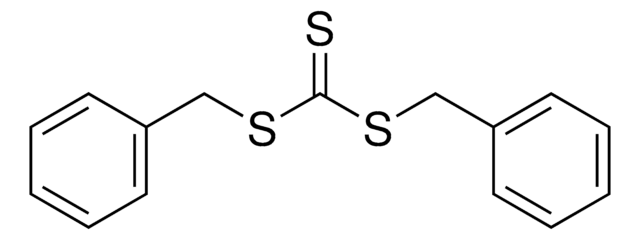
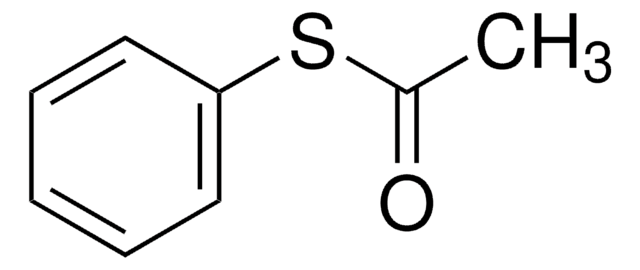
731269
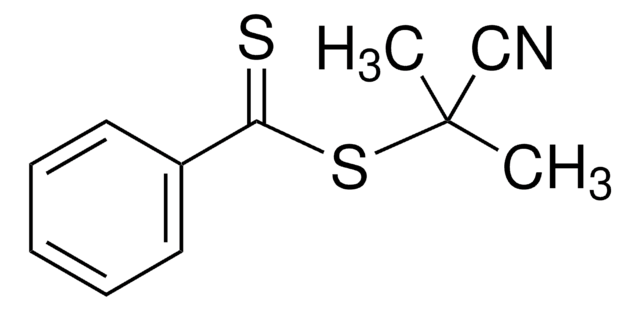
2-Phenyl-2-propyl benzodithioate
99% (HPLC)
Synonym(s):
2-Phenylpro-2-yl dithiobenzoate, Benzenecarbodithioic acid 1-methyl-1phenylethyl ester, Cumyl dithiobenzoate
About This Item
Recommended Products
Assay
99% (HPLC)
form
solid
density
1.125 g/mL at 25 °C
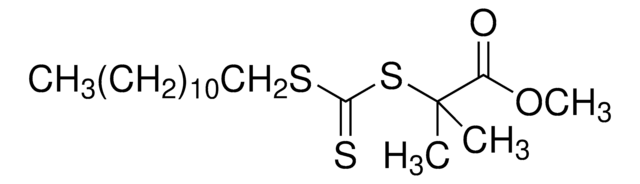
SMILES string
CC(C)(SC(=S)c1ccccc1)c2ccccc2
InChI
1S/C16H16S2/c1-16(2,14-11-7-4-8-12-14)18-15(17)13-9-5-3-6-10-13/h3-12H,1-2H3
InChI key
KOBJYYDWSKDEGY-UHFFFAOYSA-N
General description
Application
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
219.9 °F
Flash Point(C)
104.4 °C
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
A series of polymerization were carried out using RAFT agents and monomers yielding well-defined polymers with narrow molecular weight distributions. The process allows radical-initiated growing polymer chains to degeneratively transfer reactivity from one to another through the use of key functional groups (dithioesters, trithiocarbonates, xanthates and dithiocarbamates). RAFT agents help to minimize out-of-control growth and prevent unwanted termination events from occurring, effectively controlling polymer properties like molecular weight and polydispersity. RAFT agents are commercially available. RAFT does not use any cytotoxic heavy metal components (unlike ATRP).
RAFT (Reversible Addition Fragmentation chain Transfer) polymerization is a reversible deactivation radical polymerization (RDRP) and one of the more versatile methods for providing living characteristics to radical polymerization.
Over the past two decades, the rapid advance of controlled living polymerization (CLP) techniques.
The modification of biomacromolecules, such as peptides and proteins, through the attachment of synthetic polymers has led to a new family of highly advanced biomaterials with enhanced properties.
Protocols
RAFT (Reversible Addition-Fragmentation chain Transfer) is a form of living radical polymerization involving conventional free radical polymerization of a substituted monomer in the presence of a suitable chain transfer (RAFT) reagent.
We presents an article featuring procedures that describe polymerization of methyl methacrylate and vinyl acetate homopolymers and a block copolymer as performed by researchers at CSIRO.
We present an article about RAFT, or Reversible Addition/Fragmentation Chain Transfer, which is a form of living radical polymerization.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
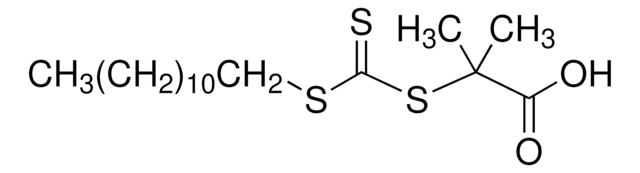
![4-Cyano-4-[(dodecylsulfanylthiocarbonyl)sulfanyl]pentanoic acid 97% (HPLC)](/deepweb/assets/sigmaaldrich/product/structures/204/925/30ae6ca0-5b0b-4963-a061-7e5e3d1a85af/640/30ae6ca0-5b0b-4963-a061-7e5e3d1a85af.png)