722081
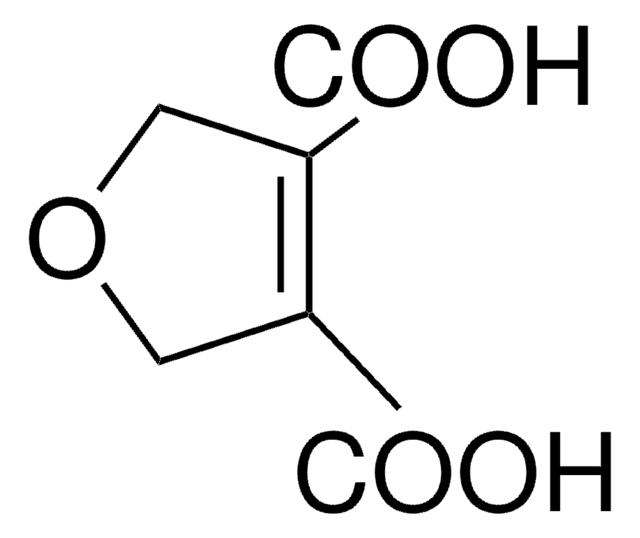
2,5-Furandicarboxylic acid
97%
Synonym(s):
Dehydromucic acid, FDCA
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H4O5
CAS Number:
Molecular Weight:
156.09
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
powder
mp
>300 °C
SMILES string
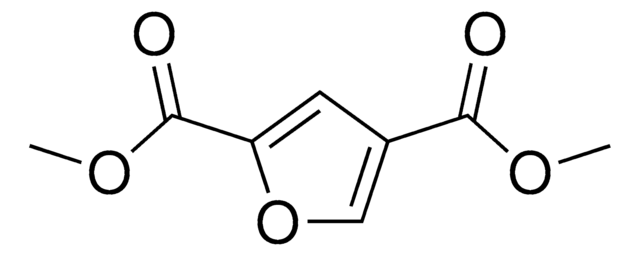
OC(=O)c1ccc(o1)C(O)=O
InChI
1S/C6H4O5/c7-5(8)3-1-2-4(11-3)6(9)10/h1-2H,(H,7,8)(H,9,10)
InChI key
CHTHALBTIRVDBM-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
2,5-Furandicarboxylic acid (FDCA) is a renewable, greener substitute for terephthalate in the production of polyesters. It is widely used as a precursor for the synthesis of bio-based polyesters and various other polymers.
Applications of FDCA in the synthesis of several metal-organic frameworks (MOFs) have also been reported.
Applications of FDCA in the synthesis of several metal-organic frameworks (MOFs) have also been reported.
Other Notes
2,5-Furandicarboxylic acid has been included among Top 10 biorefinery carbohydrate derivatives for the production of biobased industrial products.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
The furan counterpart of poly (ethylene terephthalate): An alternative material based on renewable resources.
Gandini A, et al.
Journal of Polymer Science Part A: Polymer Chemistry, 47(1), 295-298 (2009)
Polyesters derived from furan and tetrahydrofuran nuclei.
Moore J A and Kelly J E
Macromolecules, 11(3), 568-573 (1978)
Crystalline Capsules: Metal?Organic Frameworks Locked by Size?Matching Ligand Bolts.
Wang H, et al.
Angewandte Chemie (International Edition in English), 54(20), 5966-5970 (2015)
Giulia Guidotti et al.
International journal of molecular sciences, 20(9) (2019-05-06)
Biopolymers are gaining increasing importance as substitutes for plastics derived from fossil fuels, especially for packaging applications. In particular, furanoate-based polyesters appear as the most credible alternative due to their intriguing physic/mechanical and gas barrier properties. In this study, block
Niki Poulopoulou et al.
Polymers, 12(1) (2020-01-23)
Intending to expand the thermo-physical properties of bio-based polymers, furan-based thermoplastic polyesters were synthesized following the melt polycondensation method. The resulting polymers, namely, poly(ethylene 2,5-furandicarboxylate) (PEF), poly(propylene 2,5-furandicarboxylate) (PPF), poly(butylene 2,5-furandicarboxylate) (PBF) and poly(1,4-cyclohexanedimethylene 2,5-furandicarboxylate) (PCHDMF) are used in blends
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service