All Photos(1)
About This Item
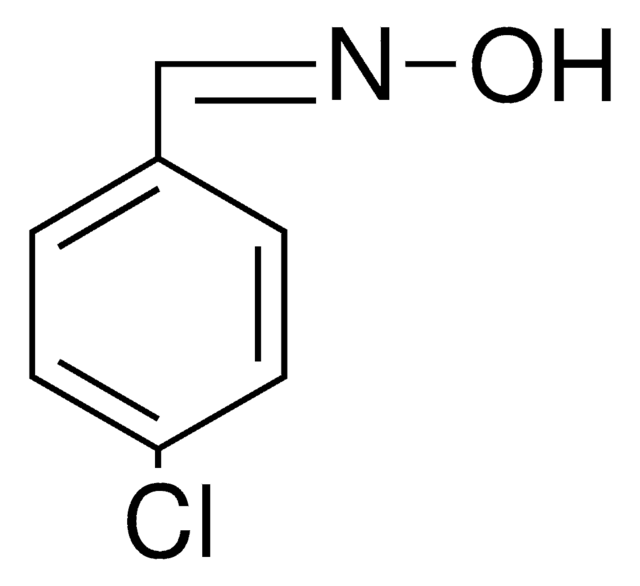
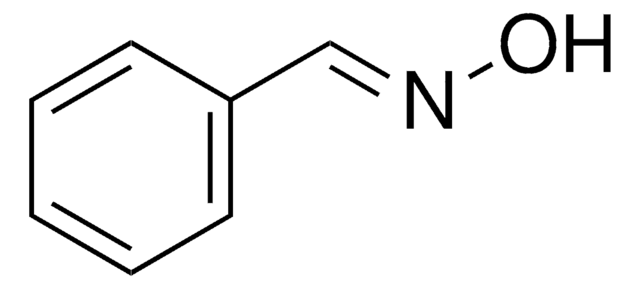
Linear Formula:
ClC6H4CH=NOH
CAS Number:
Molecular Weight:
155.58
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
mp
73-76 °C (lit.)
functional group
amine
chloro
oxime
SMILES string
O\N=C\c1ccccc1Cl
InChI
1S/C7H6ClNO/c8-7-4-2-1-3-6(7)5-9-10/h1-5,10H/b9-5+
InChI key
FZIVKDWRLLMSEJ-WEVVVXLNSA-N
Related Categories
General description
2-Chlorobenzaldehyde oxime is also known as o-chlorobenzaldehyde oxime. It can be synthesized by reacting 2-chlorobenzaldehyde and hydroxylamine hydrochloride.
Application
2-Chlorobenzaldehyde oxime may be used in the preparation of:
- 2-chlorobenzaldehyde under different reaction conditions
- methyl 3-(2-chlorophenyl)-5-[1-(4-methoxybenzyloxy)-ethyl]isoxazole-4-carboxylate
- dimethyl 3-(2-chlorophenyl)isoxazole-4,5-dicarboxylate
- [3-(2-chlorophenyl)isoxazol-5-yl]methanol
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Microwave-assisted chemoselective cleavage of oximes to their corresponding carbonyl compounds using 1, 3-dichloro-5, 5-dimethyl-hydantoin (DCDMH) as a new Deoximating reagent.
Khazaei A and Manesh AA.
Synthesis, 2005(12), 1929-1931 (2005)
Facile and Chemoselective Microwave-Assisted Cleavage of Oximes to Their Corresponding Carbonyl Compounds Using N,N?-Dibromo-N,N?-1,3-propylene-bis[(4-methylphenyl)sulfonamide] as a Deoximating Reagent.
Khazaei A, et al.
Synthesis, 2004(17), 2784-2786 (2004)
Design, synthesis and antibacterial activity of novel N-formylhydroxylamine derivatives as PDF inhibitors.
Yin L, et al.
Indian J. Chem. B, 50(5), 695-695 (2011)
Solid-phase synthesis of 5-isoxazol-4-yl-[1,2,4] oxadiazoles.
Quan C and Kurth M.
The Journal of Organic Chemistry, 69(5), 1470-1474 (2004)
A mild and selective method for the conversion of oximes into ketones and aldehydes by the use of N-bromophthalimide.
Khazaei A, et al.
J. Chem. Res. (M), 2004(10), 695-696 (2004)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service