All Photos(1)
About This Item
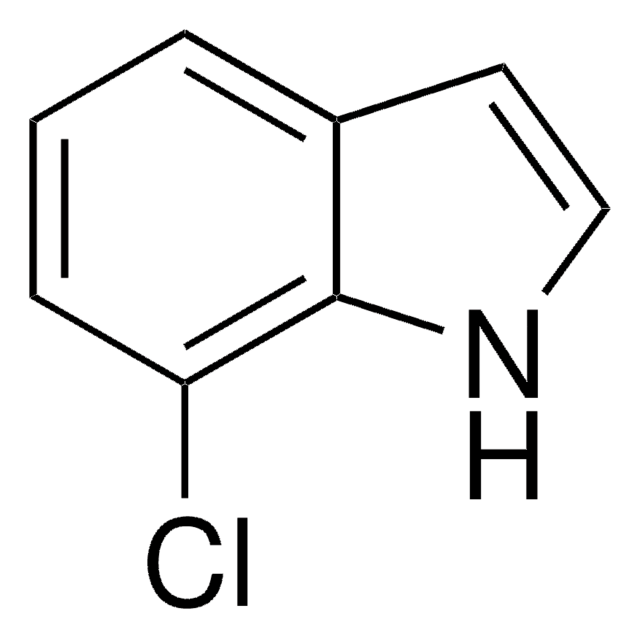
Empirical Formula (Hill Notation):
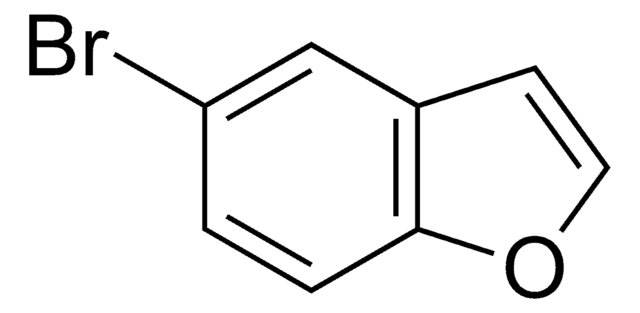
C8H6BrN
CAS Number:
Molecular Weight:
196.04
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
96%
mp
92-96 °C (lit.)
functional group
bromo
SMILES string
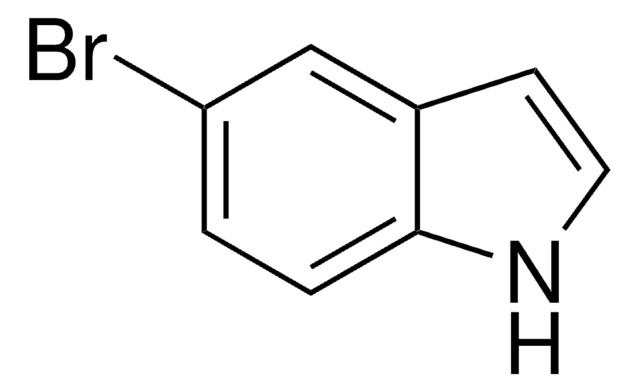
Brc1ccc2cc[nH]c2c1
InChI
1S/C8H6BrN/c9-7-2-1-6-3-4-10-8(6)5-7/h1-5,10H
InChI key
MAWGHOPSCKCTPA-UHFFFAOYSA-N
General description
6-Bromoindole is an indole derivative. It undergoes palladium-catalyzed reaction with 2-(4-fluorophenyl)ethylpiperazine to afford the carbonylation products.
Application
6-Bromoindole may be used to synthesize:
- 6-alkylthioindole
- 3-acetoxy-6-bromoindole
- 6,6′-dibromoindigo (Tyrian purple)
- 6-acylindoles
- tert-butyl 6-bromoindole-1-carboxylate
Essential starter in 6-substituted indole chemistry.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Leonardo S Santos et al.
The Journal of organic chemistry, 69(4), 1283-1289 (2004-02-14)
Described are the first enantioselective total syntheses of (+)-arborescidine A ((+)-1), (-)-arborescidine B ((-)-2), and (-)-arborescidine C ((-)-3), via routes that proceeded in five steps and 50% overall yield, eight steps and 61% overall yield, and nine steps and 51%
Palladium-catalyzed carbonylation of haloindoles: No need for protecting groups.
Kumar K, et al.
Organic Letters, 6(1), 7-10 (2004)
A facile synthesis of Tyrian purple based on a biosynthetic pathway.
Tanoue Y, et al.
Fisheries Science (Tokyo, Japan), 67(4), 726-729 (2001)
Efficient synthesis of 5-and 6-tributylstannylindoles and their reactivity with acid chlorides in the Stille coupling reaction.
Cherry K, et al.
Tetrahedron Letters, 48(33), 5751-5753 (2007)
James R Fuchs et al.
Journal of the American Chemical Society, 126(16), 5068-5069 (2004-04-22)
The first total synthesis of racemic perophoramidine is described. The key step features the highly stereoselective introduction of the vicinial quaternary centers via base-promoted carbon-carbon bond formation between a 3-alkylindole and a 3-bromo-3-alkylindolin-2-one. This transformation presumably proceeds through a conjugate
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service