All Photos(1)
About This Item
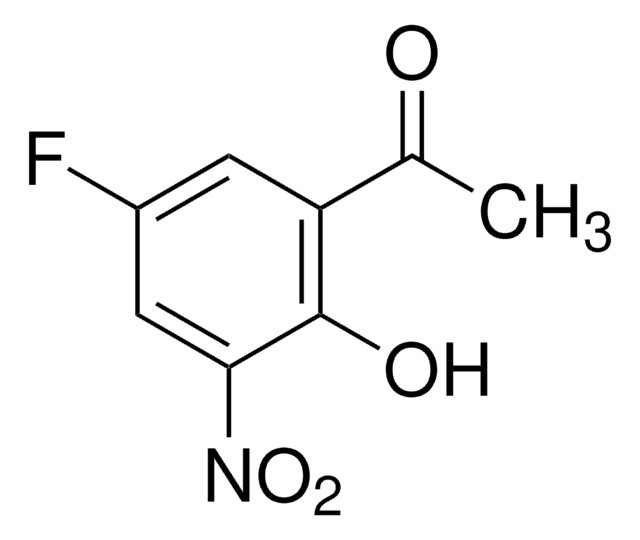
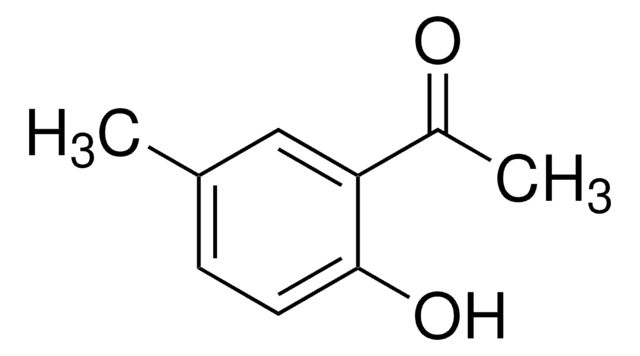
Linear Formula:
FC6H3(OH)COCH3
CAS Number:
Molecular Weight:
154.14
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
mp
31-35 °C (lit.)
functional group
fluoro
ketone
SMILES string
CC(=O)c1ccc(F)cc1O
InChI
1S/C8H7FO2/c1-5(10)7-3-2-6(9)4-8(7)11/h2-4,11H,1H3
InChI key
HLTBTUXAMVOKIH-UHFFFAOYSA-N
General description
4′-Fluoro-2′-hydroxyacetophenone is a substituted acetophenone derivative. Biological Baeyer-Villiger oxidation of 4′-fluoro-2′-hydroxyacetophenone to 4-fluorocatechol by using whole cells of Pseudomonas fluorescens ACB has been reported. Its crystals belong to the monoclinic crystal system and space group P21/n.
Application
4′-Fluoro-2′-hydroxyacetophenone may be used in the preparation of series of 4′-fluoro-2′-hydroxychalcones, via aldol condensation with substituted aldehydes followed by cyclization with hydrazine hydrate.
Used recently in the preparation of medicinally active benzo[b]furans and thiophenes.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
210.2 °F - closed cup
Flash Point(C)
99 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
4'-Fluoro-2'-hydroxyacetophenone.
Rizal MR and Ng SW.
Acta Crystallographica Section E, Structure Reports Online, 64(5), o916-o916 (2008)
Khaled R A Abdellatif et al.
Journal of enzyme inhibition and medicinal chemistry, 30(3), 484-491 (2014-09-10)
In an effort to develop safe and potent anti-inflammatory agents, a series of novel 4'-fluoro-2'-hydroxychalcones 5a-d and their dihydropyrazole derivatives 6a-d was prepared. It was synthesized via aldol condensation of 4'-fluoro-2'-hydroxyacetophenone with appropriately substituted aldehydes followed by cyclization with hydrazine
M J Moonen et al.
Journal of industrial microbiology & biotechnology, 26(1-2), 35-42 (2001-09-11)
The biological Baeyer-Villiger oxidation of acetophenones was studied by 19F nuclear magnetic resonance (NMR). The 19F NMR method was used to characterise the time-dependent conversion of various fluorinated acetophenones in either whole cells of Pseudomonas fluorescens ACB or in incubations
Journal of Heterocyclic Chemistry, 30, 445-445 (1993)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service