All Photos(1)
About This Item
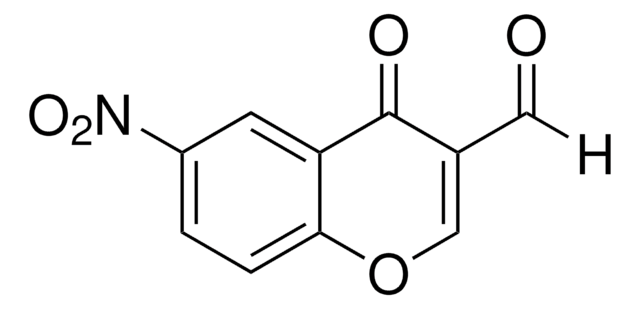
Empirical Formula (Hill Notation):
C9H5NO5
CAS Number:
Molecular Weight:
207.14
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
mp
172 °C (dec.) (lit.)
functional group
ester
ketone
nitro
SMILES string
OC1=C(C(=O)Oc2ccccc12)[N+]([O-])=O
InChI
1S/C9H5NO5/c11-8-5-3-1-2-4-6(5)15-9(12)7(8)10(13)14/h1-4,11H
InChI key
NZQAQAUWFHMVEM-UHFFFAOYSA-N
General description
4-Hydroxy-3-nitrocoumarin is a coumarin derivative and its cytotoxic action against cultured human tumor and normal cells has been investigated. It can be prepared by the nitration of 4-hydroxycoumarin in glacial acetic acid by using 72% HNO3.
Application
4-Hydroxy-3-nitrocoumarin may be used as starting reagent for the synthesis of following compounds:
- 4-chloro-3-nitrocoumarin
- 2-unsubstituted 3-nitrochromone
- 4-amino-3-nitrocoumarins
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Oral
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Biljana R Dekić et al.
Molecules (Basel, Switzerland), 15(4), 2246-2256 (2010-04-30)
Synthesis, spectral analysis and bioactivity of new coumarin derivatives are described in this paper. Eight new coumarin derivatives were synthesized in moderate to good yields by condensation of 4-chloro-3-nitrocoumarin and the corresponding heteroarylamine. The synthesized compounds were tested for their
Masami Kawase et al.
In vivo (Athens, Greece), 19(4), 705-711 (2005-07-08)
A preliminary exploration of coumarin derivatives as novel multidrug resistance (MDR) modulators was carried out to determine the basic features of the structure responsible for the MDR reversal activity. Among 44 coumarins, 14 compounds moderately induced the reversal of MDR
Vidoslav Dekić et al.
Magnetic resonance in chemistry : MRC, 48(11), 896-902 (2010-09-08)
Herein, we describe the synthesis and complete assignment of the (1)H and (13)C NMR chemical shifts of a series of antimicrobial 4-arylamino-3-nitrocoumarin derivatives based on a combination of (1)H and (13)C NMR, (1)H-(1)H-COSY, NOESY, HSQC and HMBC experiments. Conformational effects
Synthesis of heteroannulated 3-nitro-and 3-aminopyridines by cyclocondensation of electron-rich aminoheterocycles with 3-nitrochromone.
Iaroshenko VO, et al.
Tetrahedron, 68(11), 2532-2543 (2012)
Investigations of pyrans and related compounds.
Savel'ev VL, et al.
Chemistry of Heterocyclic Compounds, 9(7), 816-820 (1973)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service