414336
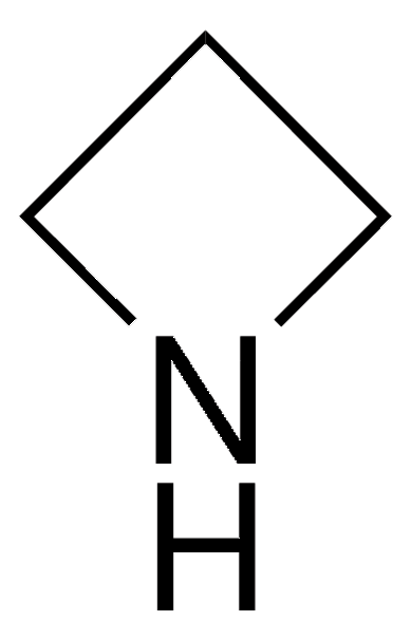
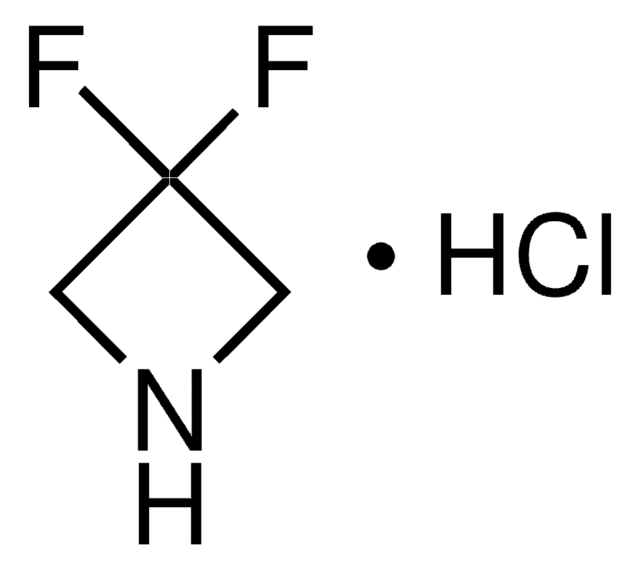
Azetidine hydrochloride
97%
Synonym(s):
Trimethyleneimine hydrochloride
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C3H7N · HCl
CAS Number:
Molecular Weight:
93.56
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
mp
>300 °C (lit.)
SMILES string
Cl[H].C1CNC1
InChI
1S/C3H7N.ClH/c1-2-4-3-1;/h4H,1-3H2;1H
InChI key
HGQULGDOROIPJN-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
305.6 °F
Flash Point(C)
152 °C
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis of ornithine lactams via diastereoselective photocyclization of 2-amino-4-oxo-4-phenyl-butanoyl amines.
Lindemann U, et al.
Tetrahedron Asymmetry, 9(24), 4459-4473 (1998)
Dongliang Chang et al.
Organic letters, 4(11), 1859-1862 (2002-05-25)
[reaction: see text] Hydroxylation of N-substituted azetidines 11 and 12 and piperidines 15-19 with Sphingomonas sp. HXN-200 gave 91-98% of the corresponding 3-hydroxyazetidines 13 and 14 and 4-hydroxypiperidines 20-24, respectively, with high activity and excellent regioselectivity. High yields and high
Christopher S Dunkley et al.
Bioorganic & medicinal chemistry letters, 13(17), 2899-2901 (2003-11-13)
A series of compounds containing an N-(4'-substituted-3'-nitrophenyl)sydnone moiety with potential antitumor activity was prepared based on active analogues. The rationale behind the design of these compounds is presented along with the 4-step synthetic route to the derivatives in the 4'-position
Valérian Gobé et al.
Organic letters, 16(20), 5438-5441 (2014-10-01)
1,5-/1,6-Allenals conjugated to an aromatic ring undergo a cyclization, in the presence of an amine, that leads to tricyclic compounds including the 1-aminotetralin scaffold. This domino process combines the in situ formation of the enamine and the cyclization affording the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service