412791
N,O-Di-Boc-hydroxylamine
97%
Synonym(s):
N,O-Bis(tert-butoxycarbonyl)hydroxylamine, tert-Butyl N-(tert-butoxycarbonyloxy)carbamate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(CH3)3COCONHOCOOC(CH3)3
CAS Number:
Molecular Weight:
233.26
Beilstein:
1794022
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
mp
67-70 °C (lit.)
functional group
amine
storage temp.
−20°C
SMILES string
CC(C)(C)OC(=O)NOC(=O)OC(C)(C)C
InChI
1S/C10H19NO5/c1-9(2,3)14-7(12)11-16-8(13)15-10(4,5)6/h1-6H3,(H,11,12)
InChI key
AGOSGCWATIJZHQ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
N,O-Di-Boc-hydroxylamine (N,O-Bis(tert-butoxycarbonyl)hydroxylamine) may be used for the synthesis of the following:
- 5-lipoxygenase inhibitor LY280810
- hydroxylamines
- hydroxamic acids,
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
A convenient method for the preparation of hydroxamic acids.
Reddy AS, et al.
Tetrahedron Letters, 41(33), 6285-6288 (2000)
A facile synthesis of N, O-bis (tert-butoxycarbonyl)-hydroxylamine.
Staszak MA and Doecke CW.
Tetrahedron Letters, 34(44), 7043-7044 (1993)
The use of N, O-bis (tert-butoxycarbonyl)-hydroxylamine in the synthesis of N-hydroxylamines and hydroxamic acids.
Staszak MA and Doecke CW.
Tetrahedron Letters, 35(23), 6021-6024 (1994)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service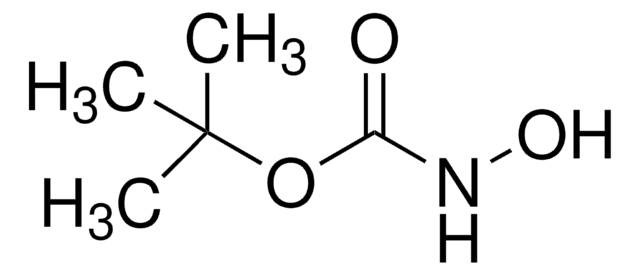


![3-[(Benzyloxycarbonyl)amino]propionaldehyde 95%](/deepweb/assets/sigmaaldrich/product/structures/408/203/100fb0f0-7072-41be-b6e0-2857cdc324ee/640/100fb0f0-7072-41be-b6e0-2857cdc324ee.png)
