410756
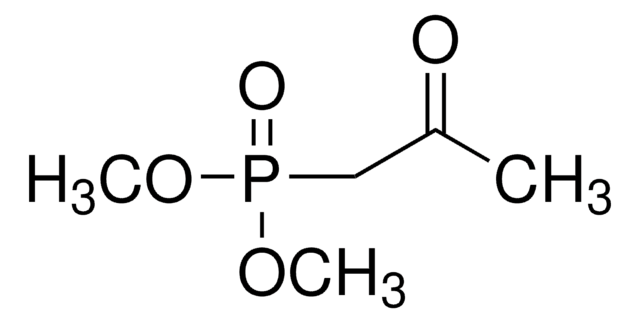
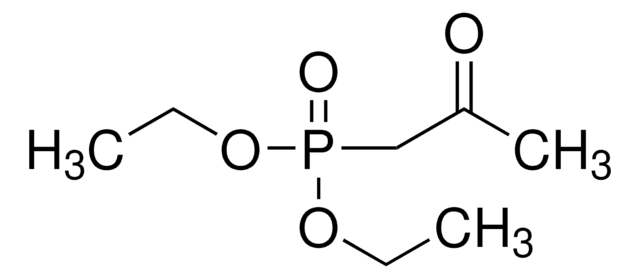
Diethyl (2-oxo-2-phenylethyl)phosphonate
97%
Synonym(s):
Diethyl benzoylmethylphosphonate, Diethyl phenacylphosphonate, NSC 648426
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
C6H5COCH2P(O)(OC2H5)2
CAS Number:
Molecular Weight:
256.23
MDL number:
UNSPSC Code:
12352108
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
reaction suitability
reaction type: C-C Bond Formation
refractive index
n20/D 1.513 (lit.)
bp
192-193 °C/11 mmHg (lit.)
density
1.179 g/mL at 25 °C (lit.)
functional group
ketone
phenyl
phosphonate
SMILES string
CCOP(=O)(CC(=O)c1ccccc1)OCC
InChI
1S/C12H17O4P/c1-3-15-17(14,16-4-2)10-12(13)11-8-6-5-7-9-11/h5-9H,3-4,10H2,1-2H3
InChI key
HPEVTTNSIPGLEL-UHFFFAOYSA-N
Application
Reactant involved in:
- Asymmetric Michael addition of β-oxo phosphonates to nitro olefins
- Gem-chlorofluorination of keto phosphonates with subsequent functionalization of the products
- Cyclocondensation reactions to produce arylphosphonates
- Diazo transfer reactions for synthesis of diazo-phosphonyl compounds
- Horner-Wadsworth-Emmons reactions
- Inverse-electron-demand Diels-Alder reactions
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Douglass F Taber et al.
Tetrahedron letters, 49(48), 6904-6906 (2009-12-01)
An convenient reagent for the one-carbon homologation of an aldehyde to the corresponding alkyne is reported. This reagent allows this conversion to conveniently be carried out on a large scale under ambient conditions.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service