All Photos(1)
About This Item
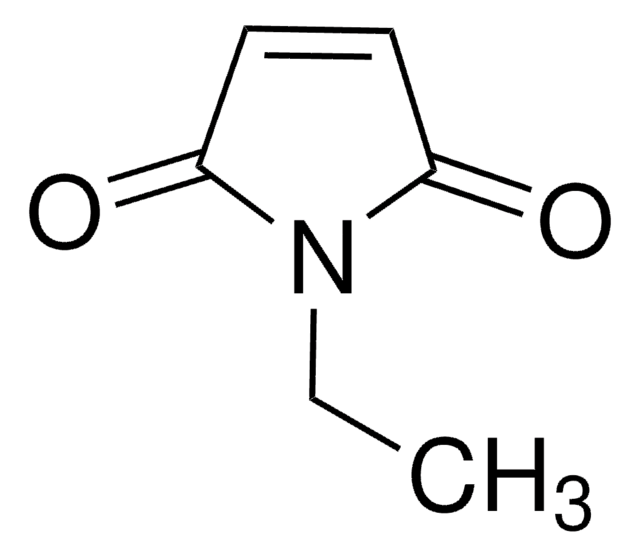
Empirical Formula (Hill Notation):
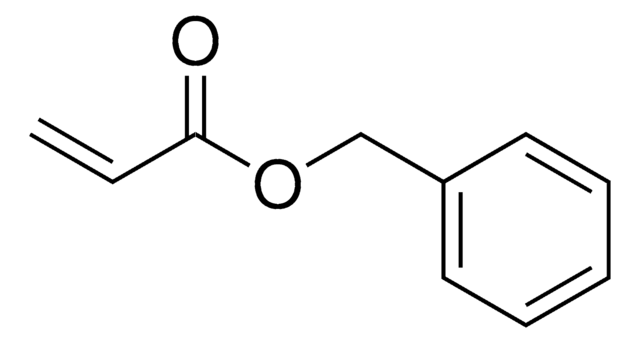
C11H9NO2
CAS Number:
Molecular Weight:
187.19
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
solid
mp
70 °C (lit.)
functional group
imide
maleimide
phenyl
SMILES string
O=C1C=CC(=O)N1Cc2ccccc2
InChI
1S/C11H9NO2/c13-10-6-7-11(14)12(10)8-9-4-2-1-3-5-9/h1-7H,8H2
InChI key
MKRBAPNEJMFMHU-UHFFFAOYSA-N
Related Categories
General description
N-Benzylmaleimide is an N-substituted maleimide and its microwave-mediated facile and fast synthesis has been described. Radical and anionic copolymerization of N-benzylmaleimide with optically active N-(L-menthoxycarbonylmethyl)maleimide has been reported. Base-catalyzed asymmetric cycloaddition of anthrone with N-benzylmaleimide was studied in the presence of C2-chiral pyrrolidines. Stereoselctive iridium-catalyzed double incorporation reaction of styrene with N-benzylmaleimide affords cyclic product.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Microwave-induced One-pot Synthesis of N-carboxyalkyl Maleimides and Phthalimides.
Borah N, et al.
J. Chem. Res. Synop., 5, 272-273 (1998)
Construction of a single-chain Fv from an antibody which catalyses a Diels Alder cycloaddition.
L Brooks et al.
Biochemical Society transactions, 24(2), 313S-313S (1996-05-01)
Copolymerization of optically active N-(l-menthoxycarbonylmethyl) maleimide with N-phenyl-or N-benzylmaleimide.
Kagawa K, et al.
Polymer, 36(5), 941-948 (1995)
Asymmetric cycloaddition of anthrone with N-substituted maleimides with C2-chiral pyrrolidines.
Tokioka K, et al.
Tetrahedron Asymmetry, 8(1), 101-107 (1997)
X Wang et al.
Organic letters, 2(22), 3509-3512 (2000-11-18)
[reaction: see text] A new strategy for the synthesis and purification of synthetic intermediates is described using anthracene-tagged ester substrates in conjunction with an N-benzylmaleimide resin. Anthracene chemical tags permit use of standard solution-phase reaction conditions and reaction-monitoring techniques.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service