277681
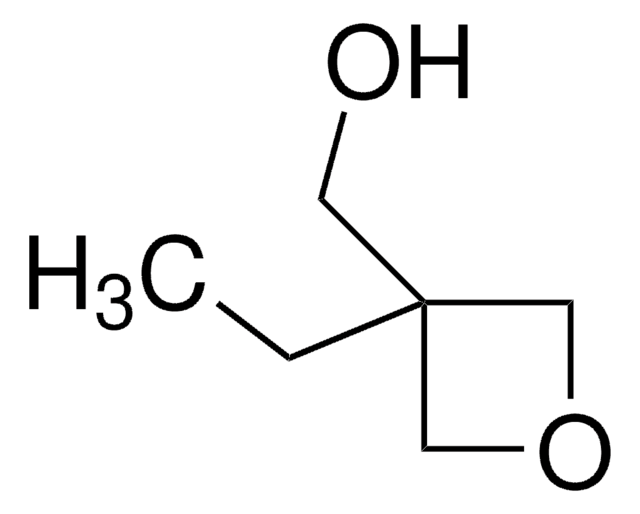
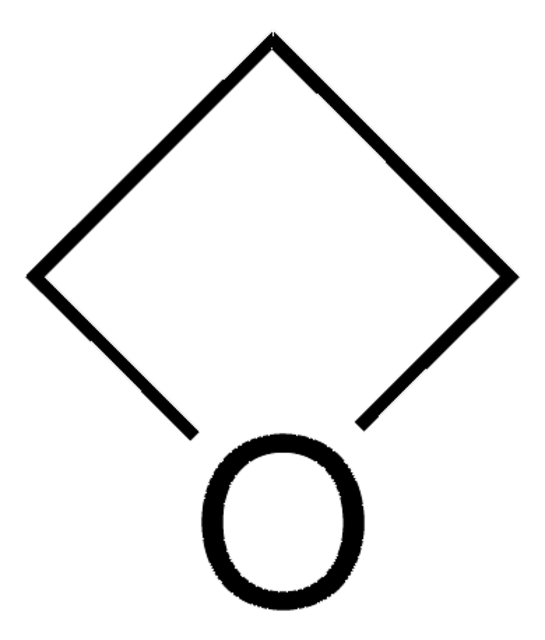
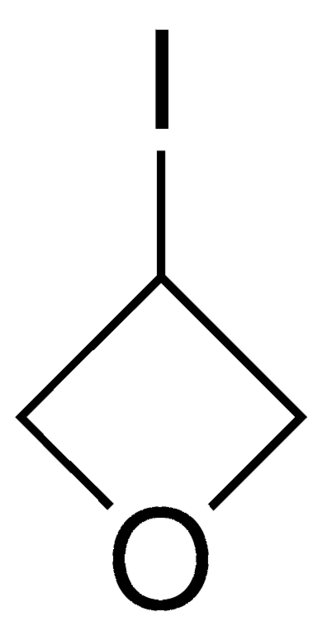
3-Methyl-3-oxetanemethanol
98%
Synonym(s):
3-Hydroxymethyl-3-methyl-oxetane
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C5H10O2
CAS Number:
Molecular Weight:
102.13
Beilstein:
102773
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
liquid
refractive index
n20/D 1.446 (lit.)
bp
80 °C/40 mmHg (lit.)
density
1.024 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
CC1(CO)COC1
InChI
1S/C5H10O2/c1-5(2-6)3-7-4-5/h6H,2-4H2,1H3
InChI key
NLQMSBJFLQPLIJ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
3-Methyl-3-oxetanemethanol has been used in the preparation of:
- star-shaped copolymer consisting of a hyperbranched poly(3-methyl-3-oxetanemethanol) core and polytetrahydrofuran arms
- pyridyl disulfide-functionalized cyclic carbonate monomer, required for the synthesis of of functional poly(ε-caprolactone) containing pendant pyridyl disulphide groups
- δ-lactams
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
206.6 °F
Flash Point(C)
97 °C
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis of a Star-Shaped Copolymer with a Hyperbranched Poly (3-methyl-3-oxetanemethanol) Core and Tetrahydrofuran Arms by One-Pot Copolymerization.
Hou J and Yan D.
Macromolecular Rapid Communications, 23(8), 456-459 (2002)
Functional poly (e-caprolactone) s via copolymerization of e-caprolactone and pyridyl disulfide-containing cyclic carbonate: controlled synthesis and facile access to reduction-sensitive biodegradable graft copolymer micelles.
Chen W, et al.
Macromolecules, 46(3), 699-707 (2013)
Bhooma Raghavan et al.
The Journal of organic chemistry, 71(5), 2151-2154 (2006-02-25)
A concise, stereoselective synthesis of alpha-substituted gamma-lactams is reported. gamma-Lactam scaffolds 2 and 3, possessing an Evans' chiral auxiliary and two types of N substituents, were successfully alkylated with different electrophiles to give alpha-substituted gamma-lactams with reasonable diastereoselectivities. The use
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service