263567
1,4-Bis(trimethylsilyl)butadiyne
98%, stable crystalline form of butadiyne
Synonym(s):
1,4-Bis(trimethylsilyl)-1,3-butadiyne, BTMSBD
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
(CH3)3SiC≡CC≡CSi(CH3)3
CAS Number:
Molecular Weight:
194.42
Beilstein:
1758268
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
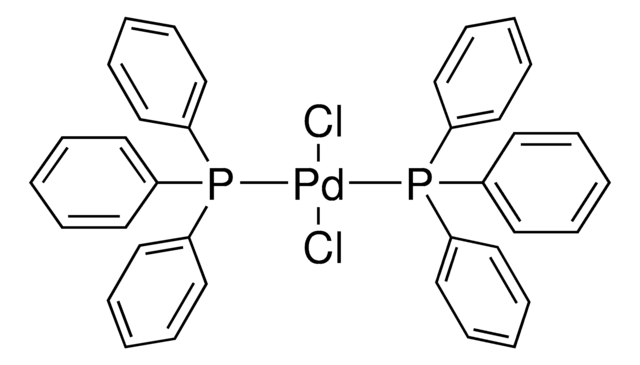
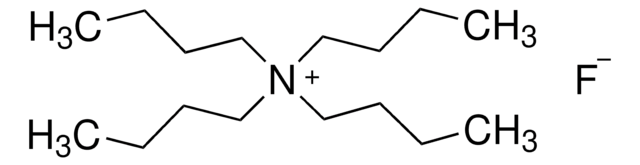
Recommended Products
Assay
98%
form
solid
refractive index
n20/D 1.384 (lit.)
mp
111-113 °C (lit.)
density
0.974 g/mL at 20 °C (lit.)
storage temp.
2-8°C
SMILES string
C[Si](C)(C)C#CC#C[Si](C)(C)C
InChI
1S/C10H18Si2/c1-11(2,3)9-7-8-10-12(4,5)6/h1-6H3
InChI key
LBNVCJHJRYJVPK-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
1,4-Bis(trimethylsilyl)butadiyne can be used as a reagent to prepare:
- 1,1,3,4-Tetrasilyl-substituted 1,3-butadienes or 1,1,3,4-tetrasilyl-substituted 1,2-butadienes by hydrosilylation reaction using various hydridosilanes and catalysts.
- Glycosylated oligo(ethynylene)s using the Negishi reaction.
- (±) Falcarinol, a polyacetylene class of fatty alcohol.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Sol. 1
Storage Class Code
4.1B - Flammable solid hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Tobias N Hoheisel et al.
Organic letters, 10(20), 4525-4528 (2008-09-25)
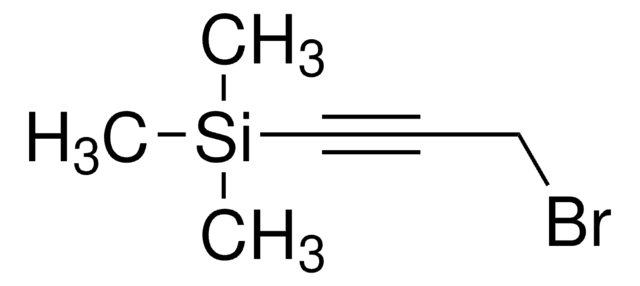
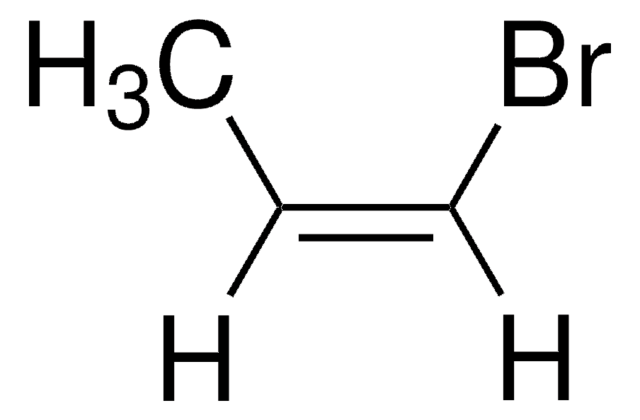
A convenient and efficient sp-sp carbon heterocoupling protocol based on the Negishi reaction was developed, in which the required zinc diacetylide was generated from 1,4-bis(trimethylsilyl)butadiyne in situ and reacted with a bromoacetylene in apolar solvent mixtures. The method has been
Manuel M Bentlohner et al.
Angewandte Chemie (International ed. in English), 54(12), 3748-3753 (2015-02-04)
The accessibility of triads with deltahedral Zintl clusters in analogy to fullerene-linker-fullerene triads is another example for the close relationship between fullerenes and Zintl clusters. The compound {[K(2.2.2-crypt)]4[RGe9-CH=CH-CH=CH-Ge9R]}(toluene)2 (R=(2Z,4E)-7-amino-5-aza-hepta-2,4-dien-2-yl), containing two deltahedral [Ge9] clusters linked by a conjugated (1Z,3Z)-buta-1,3-dien-1,4-diyl bridge
Manuel M Bentlohner et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 23(67), 17089-17094 (2017-09-15)
We report on the synthesis and structure, as well as on the mechanism of formation of the [Ge
A short synthesis of (+) and (-)-falcarinol
McLaughlin NP, et al.
Tetrahedron, 66, 9681-9687 (2010)
Hydrosilylation of 1, 4-Bis (trimethylsilyl) butadiyne and Silyl-Substituted Butenynes.
Kusumoto T, et al.
Bulletin of the Chemical Society of Japan, 65(5), 1280-1290 (1992)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service

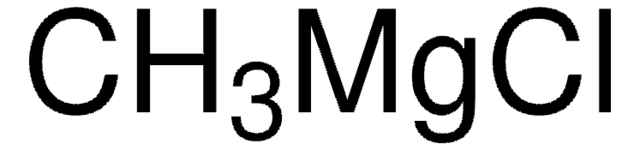

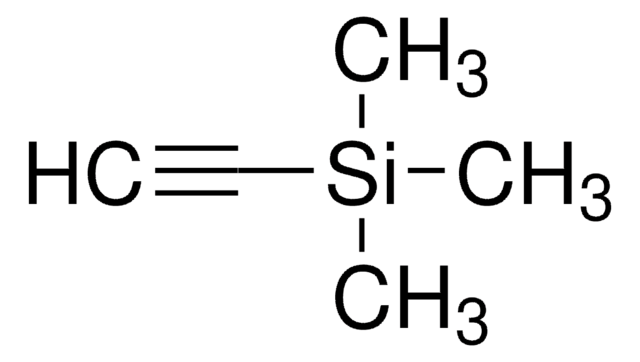


![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)