258717
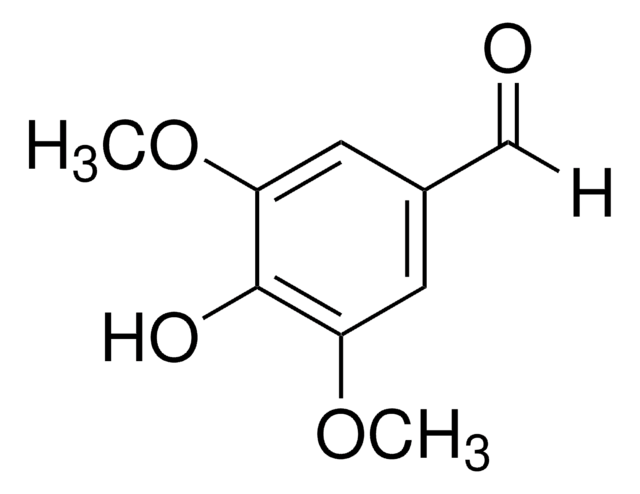
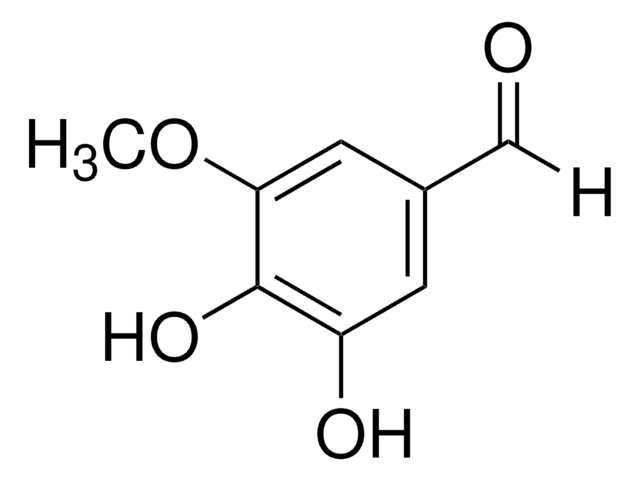
3,4-Dimethoxy-5-hydroxybenzaldehyde
98%
Synonym(s):
5-Hydroxyveratraldehyde
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
HOC6H2(OCH3)2CHO
CAS Number:
Molecular Weight:
182.17
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
powder
bp
177-180 °C/12 mmHg (lit.)
mp
67-69 °C (lit.)
functional group
aldehyde
SMILES string
COc1cc(C=O)cc(O)c1OC
InChI
1S/C9H10O4/c1-12-8-4-6(5-10)3-7(11)9(8)13-2/h3-5,11H,1-2H3
InChI key
NVLTWXMZECWWPC-UHFFFAOYSA-N
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Carsten Höltke et al.
Bioorganic & medicinal chemistry, 17(20), 7197-7208 (2009-09-22)
The expression and function of endothelin (ET) receptors is abnormal in cardiovascular diseases, tumor progression, and tumor metastasis. In this study, we prepared two [(18)F]-fluorinated derivatives of the non-peptide ET(A) receptor antagonist PD 156707 and evaluated their ET receptor binding
Timothy J Tschaplinski et al.
Biotechnology for biofuels, 5(1), 71-71 (2012-09-25)
Down-regulation of the caffeic acid 3-O-methyltransferase EC 2.1.1.68 (COMT) gene in the lignin biosynthetic pathway of switchgrass (Panicum virgatum) resulted in cell walls of transgenic plants releasing more constituent sugars after pretreatment by dilute acid and treatment with glycosyl hydrolases
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service